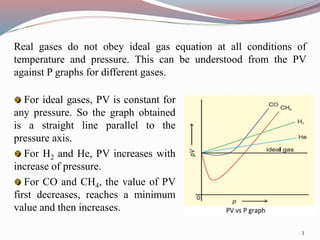
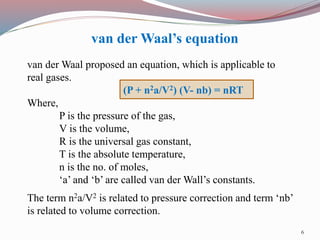
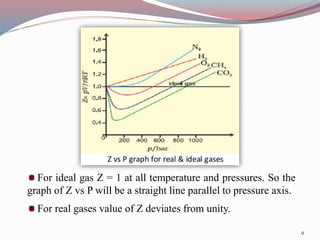
Real gases deviate from ideal gas behavior at high pressures and low temperatures due to the assumptions of negligible molecular volume and no intermolecular forces being incorrect in those conditions. Van der Waals proposed an equation to account for these deviations that includes pressure and volume correction terms related to intermolecular attractive forces and molecular size. The compressibility factor Z, which is the ratio of PV to nRT, can quantify this deviation from ideal behavior for real gases as it equals 1 for ideal gases but varies from 1 for real gases.