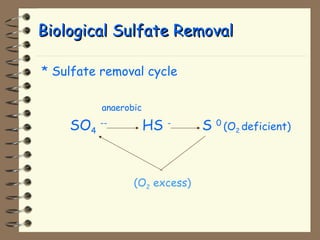
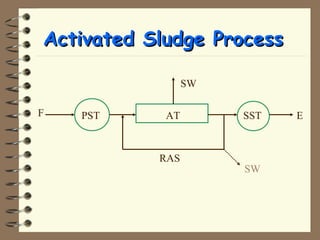
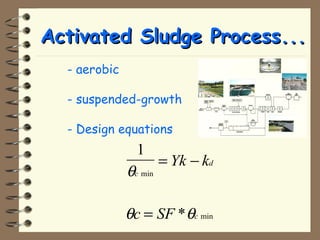
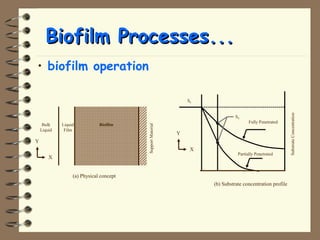
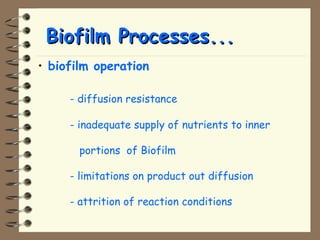
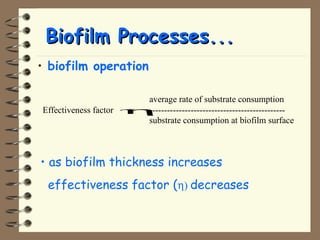
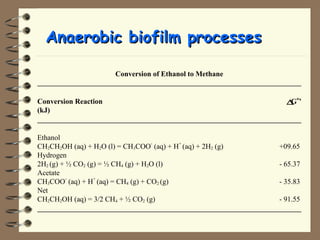
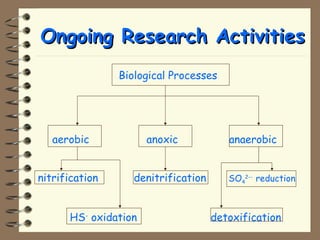
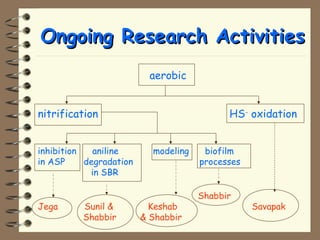
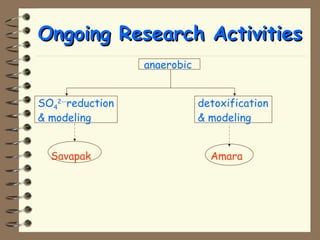
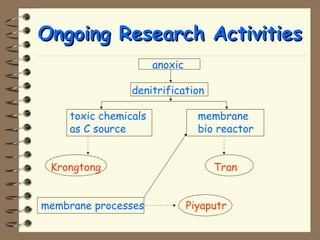
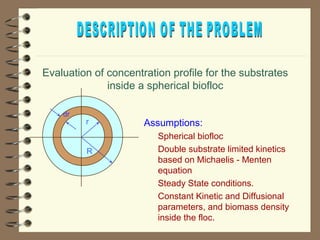
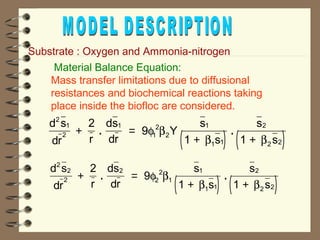
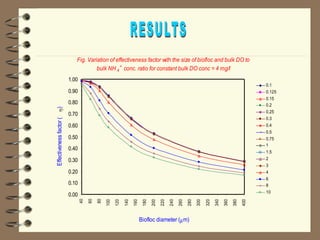
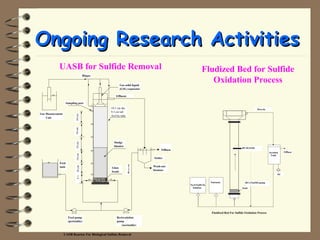
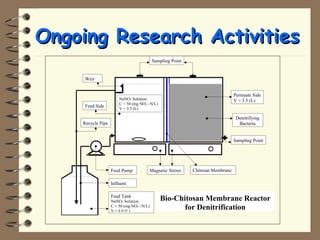
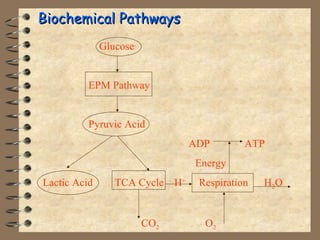
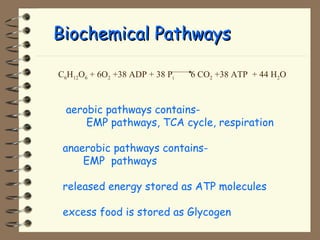
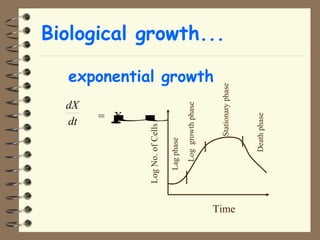
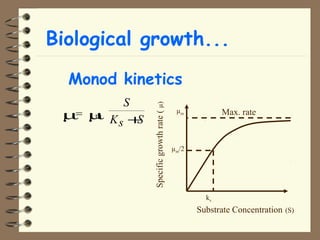
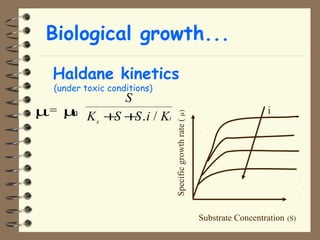
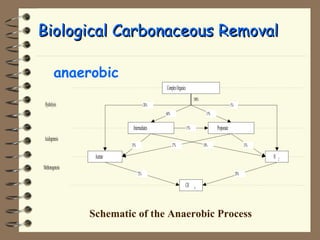
The document summarizes a seminar on biological wastewater treatment processes, past, present, and future. It discusses various types of domestic and industrial wastewater and their characteristics. It then describes key biological processes involved in wastewater treatment like carbonaceous removal, nitrogen removal, and sulfate removal. Various treatment processes are discussed including pond treatment, activated sludge process, and biofilm processes. Ongoing research activities at the institute are also highlighted which include studies on nitrification kinetics, anaerobic sulfate reduction modeling, and membrane bioreactor processes.














![BBiioollooggiiccaall NNiittrrooggeenn RReemmoovvaall
factors affecting nitrification
* temperature
* substrate concentration
* dissolved oxygen
* pH
* toxic and inhibitory substances
é
ù
NH N T
N O
m = m - -
( 0.095( 15) )[1 0.83(7.2 )]
DO
4 e pH
é
m K NH úû
- - N
K DO
4
ù
êë
+
× úû
êë
+ -](https://image.slidesharecdn.com/biologicalwastewatertreatmentprocesses-140920141105-phpapp01/85/Biological-wastewater-treatment-processes-22-320.jpg)