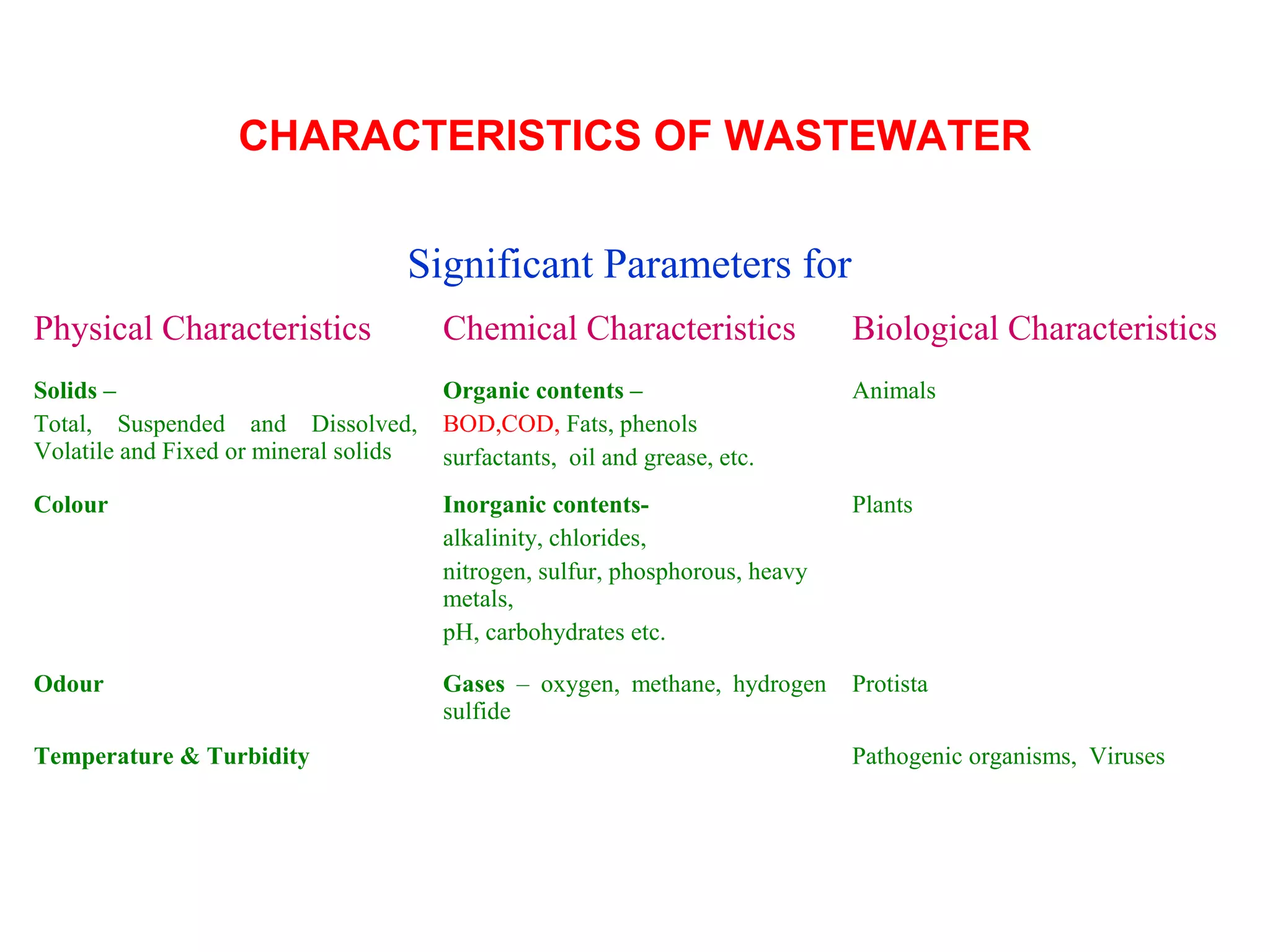
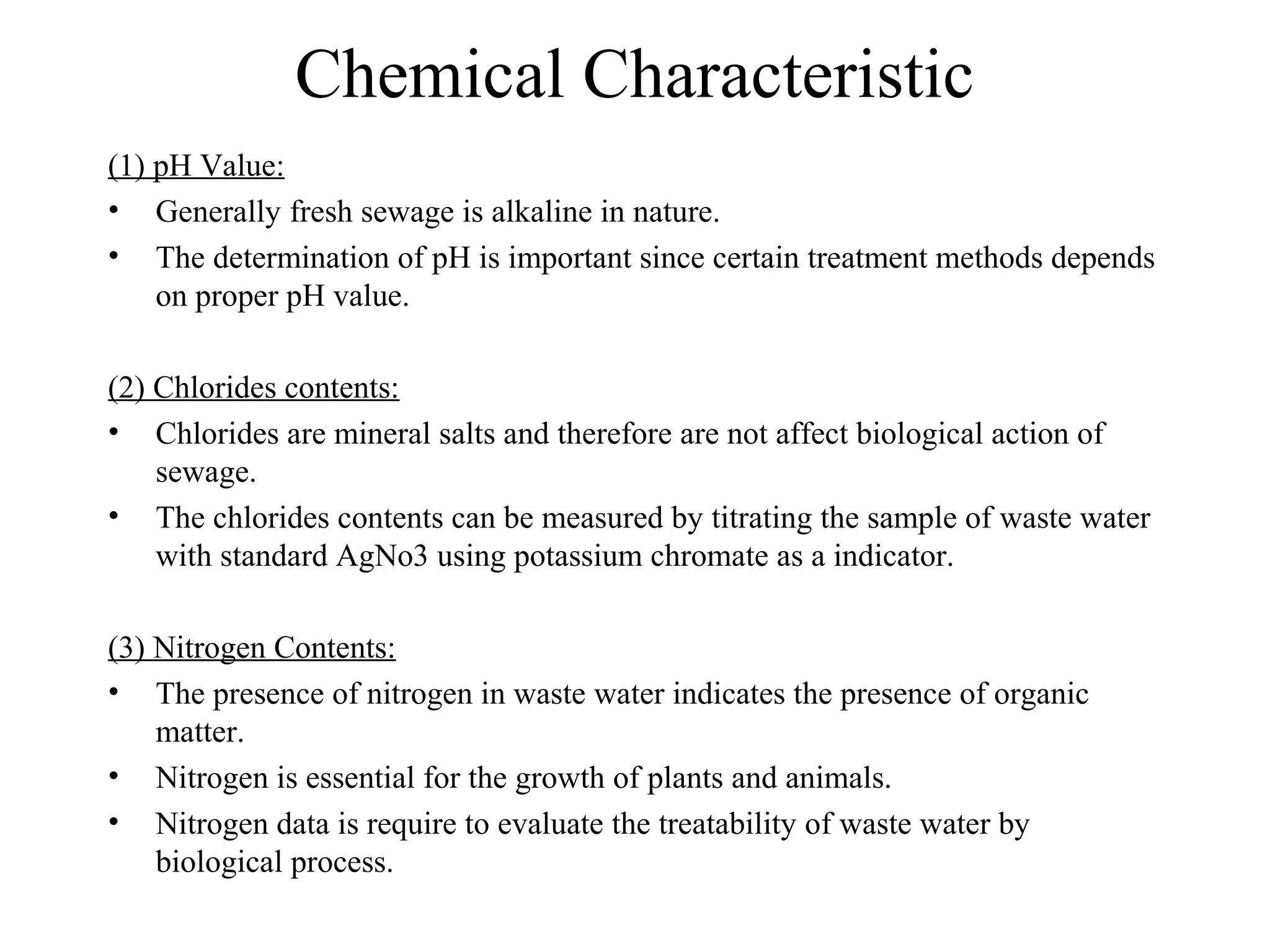
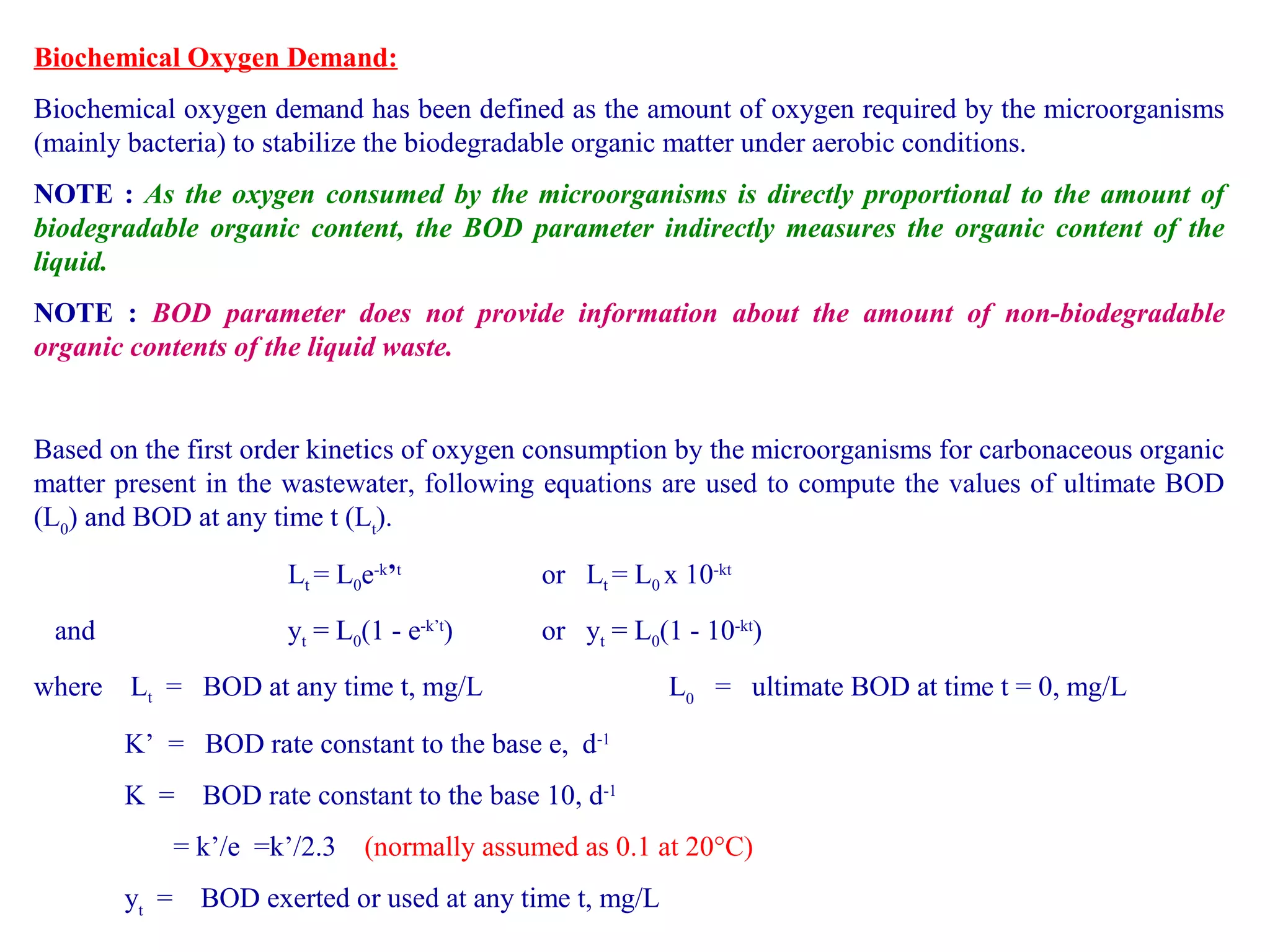
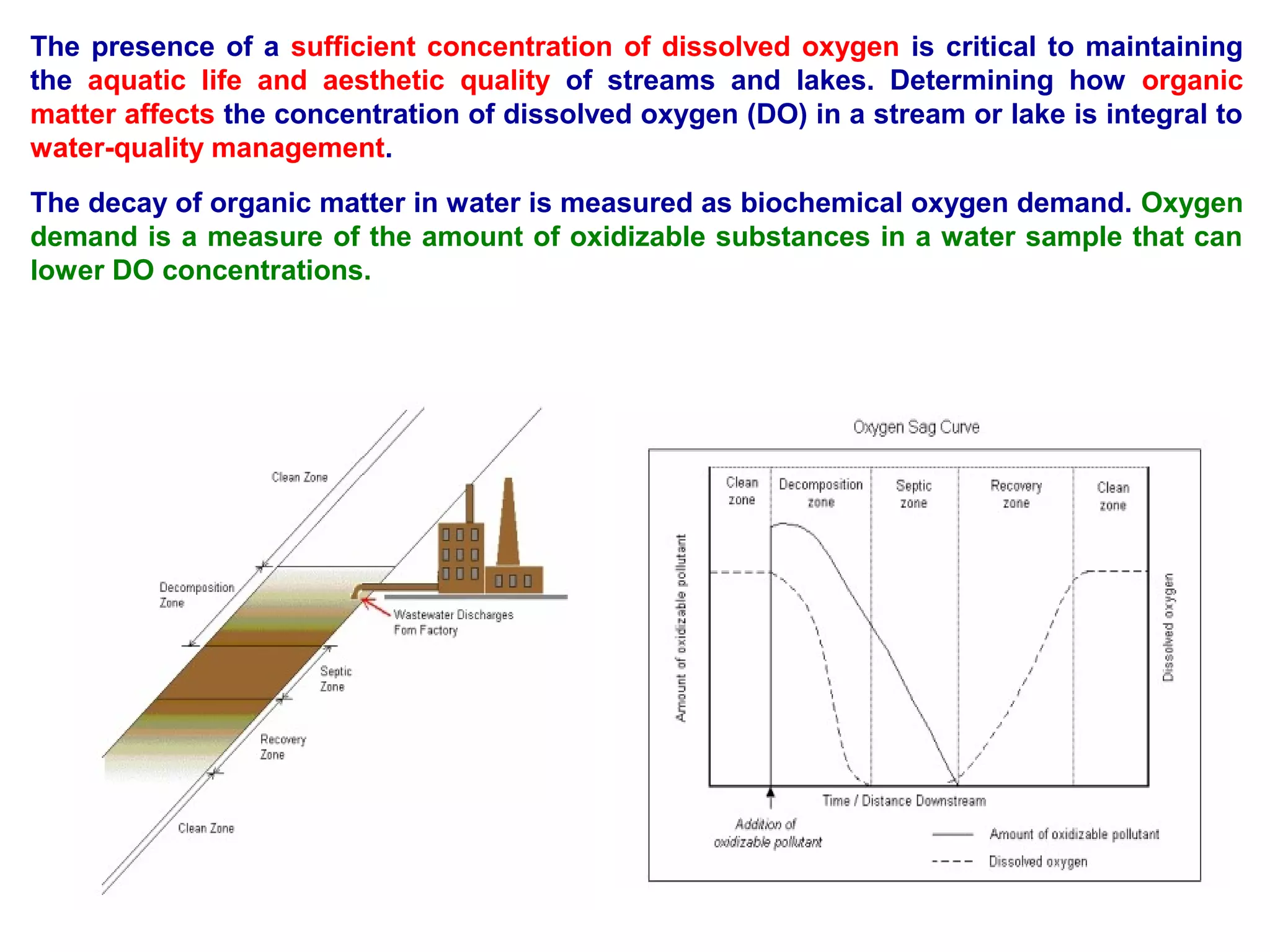
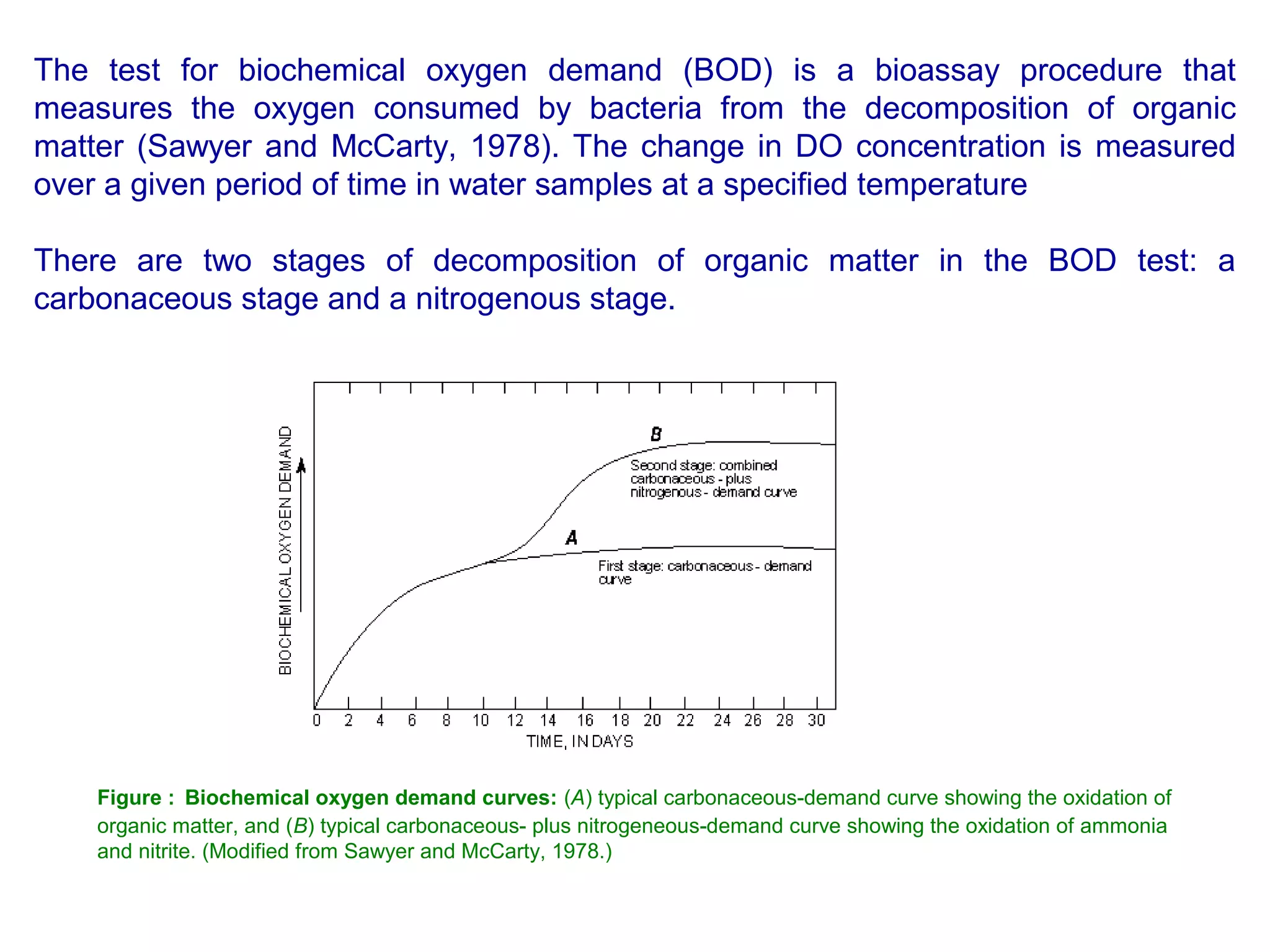
This document discusses parameters for analyzing waste water quality. It describes the objectives of waste water analysis which include monitoring treatment plant efficiency. Physical analyses examine characteristics like color and odor, while chemical analyses determine substance amounts. Key parameters discussed include biochemical oxygen demand (BOD), chemical oxygen demand (COD), dissolved oxygen, pH, nitrogen, and solids. BOD testing measures oxygen consumed by bacteria breaking down organic matter over time. COD testing uses chemical oxidization to similarly assess ability to consume oxygen. Their ratio provides information on a waste water's biodegradability.