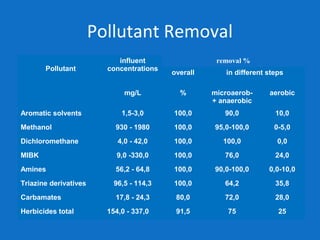
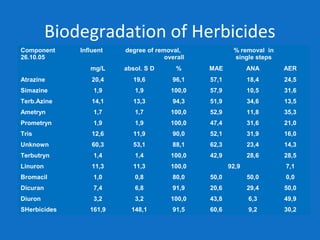
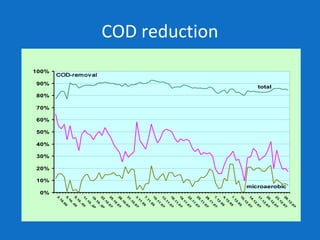
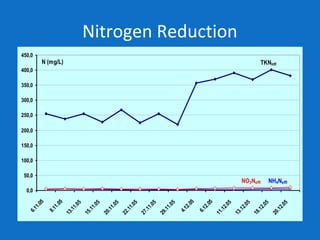
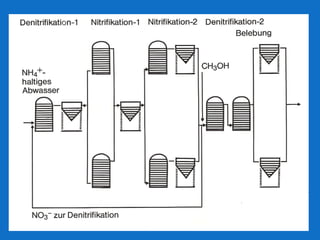
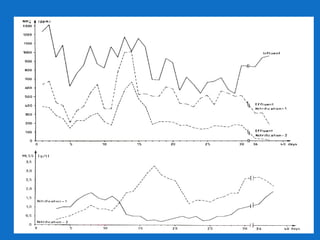
Levapor is an innovative organization specializing in bioprocess improvement for treating high-strength industrial and municipal wastewater, particularly through nitrification and denitrification techniques. They develop tailored bio carriers to enhance microbial efficiency, facilitating the removal of complex pollutants effectively. The technology demonstrated significant advantages in process stability, performance, and lower energy consumption compared to traditional systems.