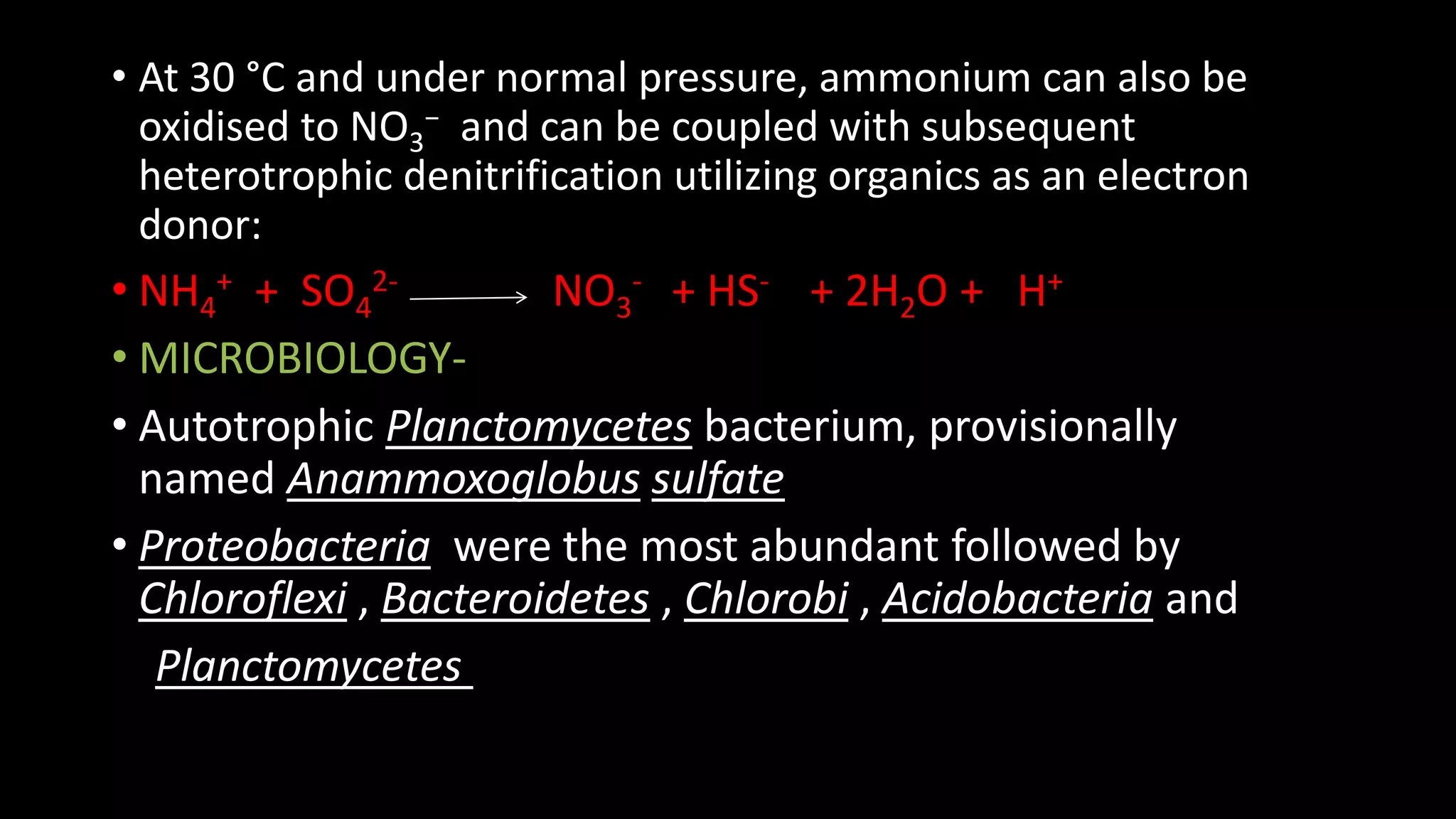
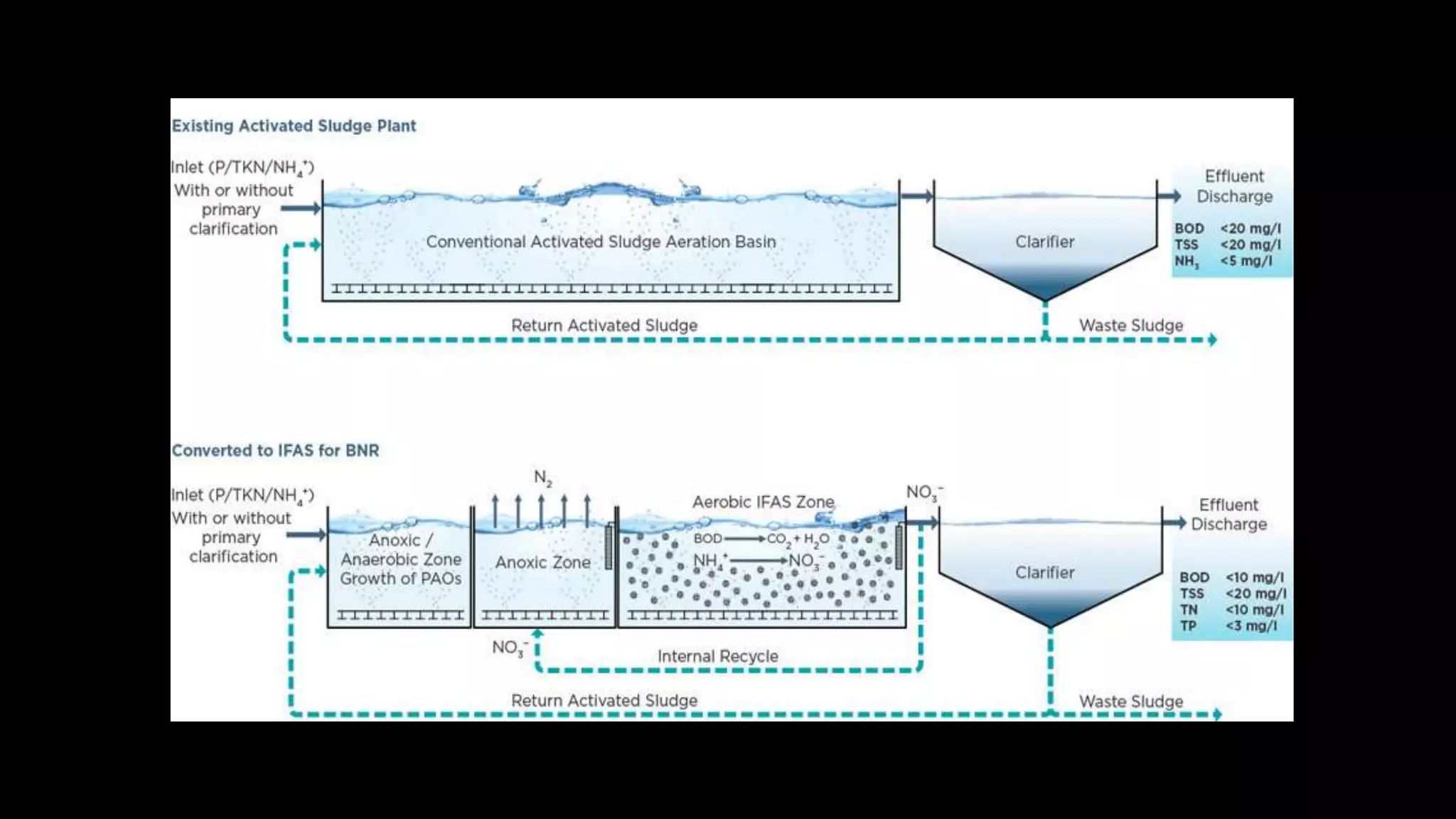
The document discusses various methods for removing nitrogen from wastewater, including biological, chemical, and physicochemical approaches. Biologically, nitrification and denitrification can convert nitrogenous wastes to nitrogen gas. Chemically, methods like breakpoint chlorination and struvite precipitation are used. Physicochemically, ammonia stripping releases nitrogen gas from wastewater. The preferred approach is nitrogen removal via nitrification and denitrification during secondary wastewater treatment using activated sludge or other suspended growth systems.