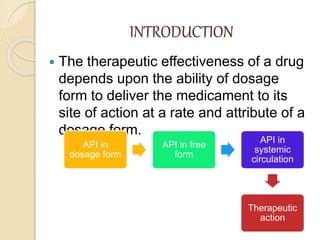
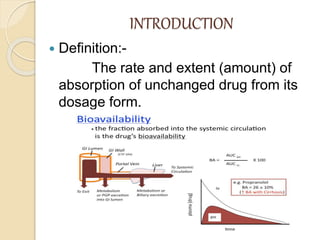
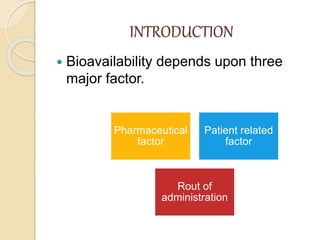
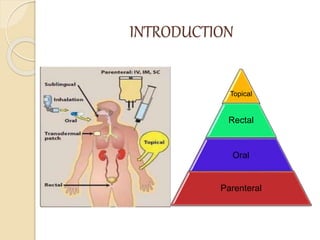
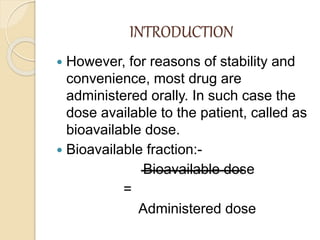
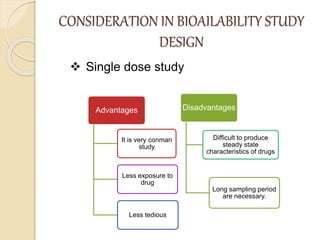
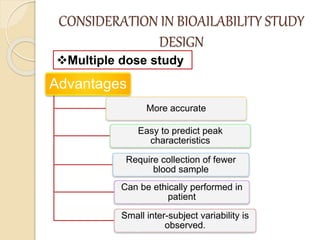
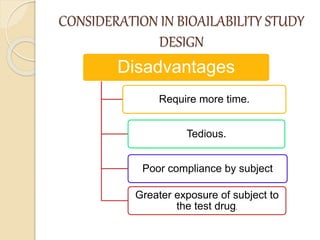
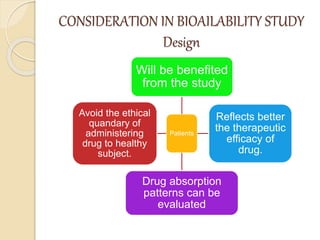
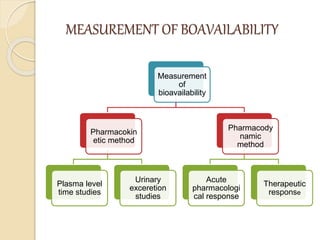
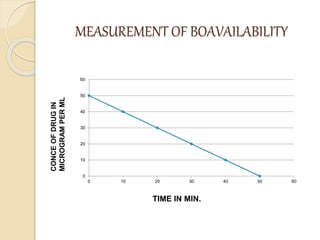
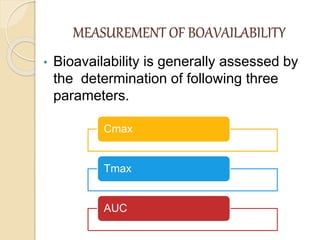
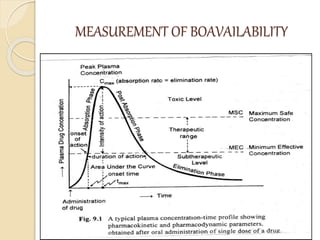
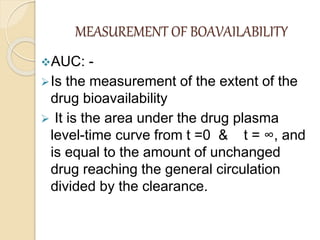
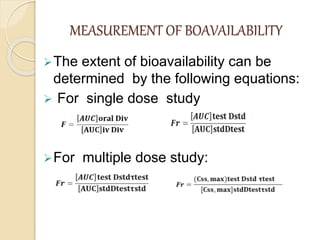
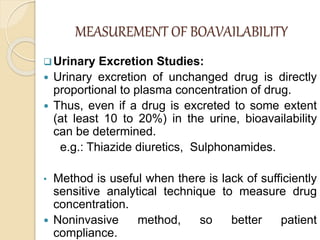
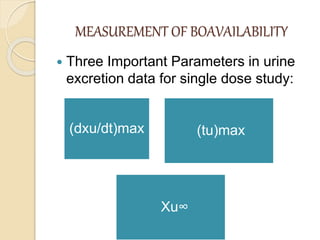
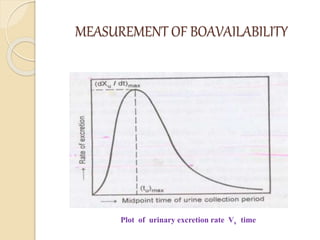
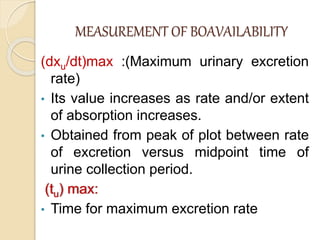
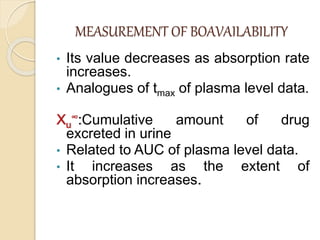
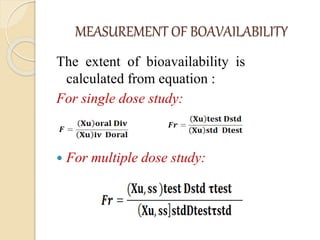
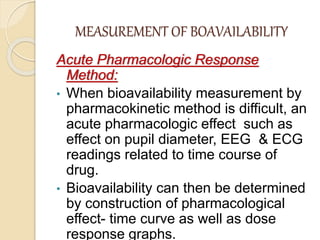
Bioavailability refers to the rate and extent of absorption of a drug from its dosage form. It depends on pharmaceutical, patient, and route of administration factors. There are two main methods to measure bioavailability - pharmacokinetic and pharmacodynamic. Pharmacokinetic methods include plasma level time studies and urinary excretion studies which analyze parameters like Cmax, Tmax, and AUC. These provide an indirect assessment of bioavailability. Pharmacodynamic methods evaluate acute pharmacological responses or therapeutic responses but are more variable. In vitro dissolution studies can also provide some indication of in vivo bioavailability.