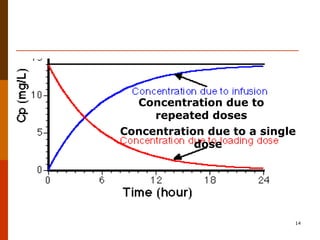
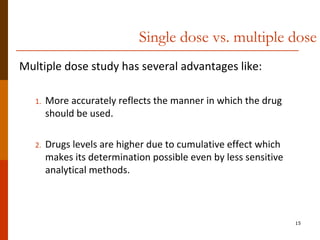
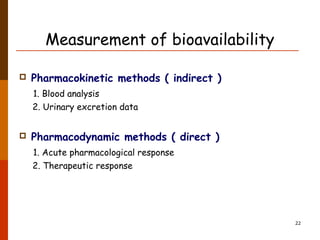
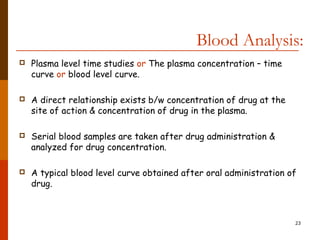
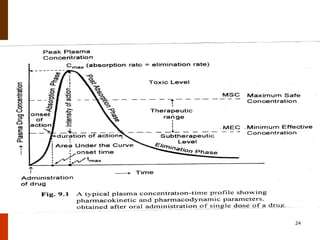
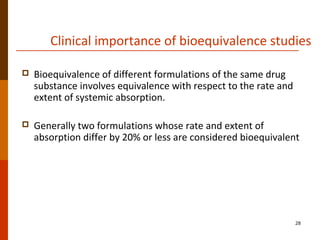
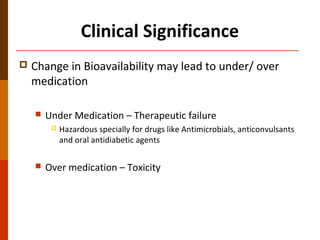
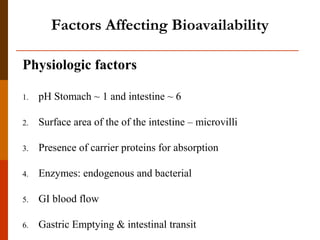
Bioavailability refers to the amount of drug that enters systemic circulation and is available at the site of action. It depends on the rate and extent of drug absorption from its dosage form. Many physiological and drug-specific factors can affect bioavailability such as pH, enzymes, blood flow, and food interactions. Careful evaluation and standardization of bioavailability is important for drug development and quality control to ensure accurate and safe dosing.