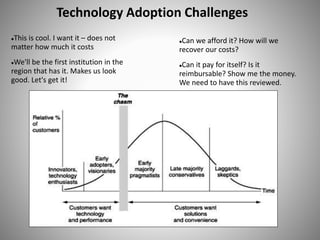
The document discusses biobusiness and biosafety, providing definitions and opportunities for biotechnology in developing countries. It examines the market for biobusiness, key opportunity areas, and factors for successful bioenterprise innovation including focusing on high-value opportunities, recognizing that innovation need not have long life cycles, and emphasizing people over technologies. The document also outlines biosafety levels and concepts from containment to facility design to protect laboratory workers and the environment.