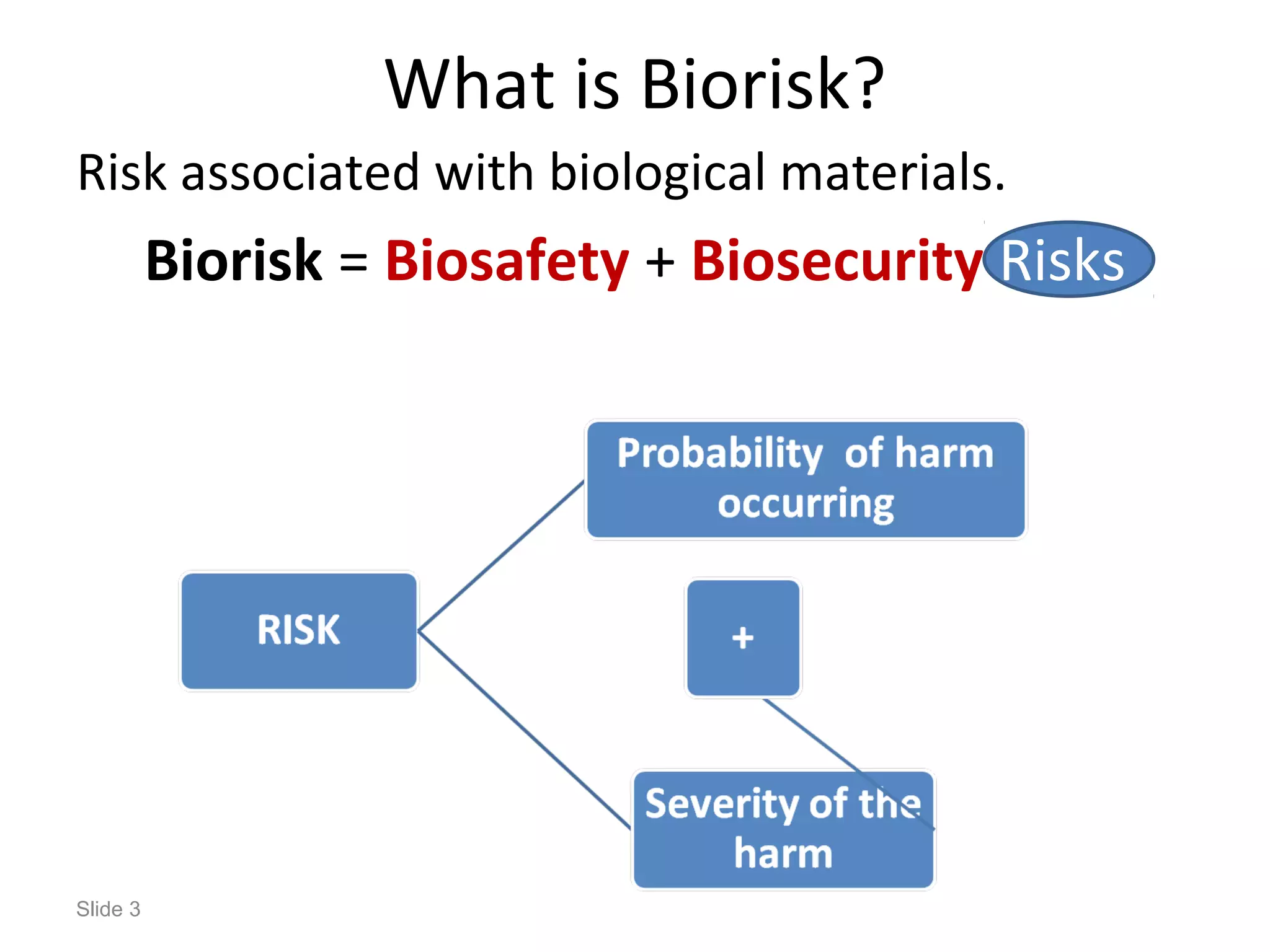
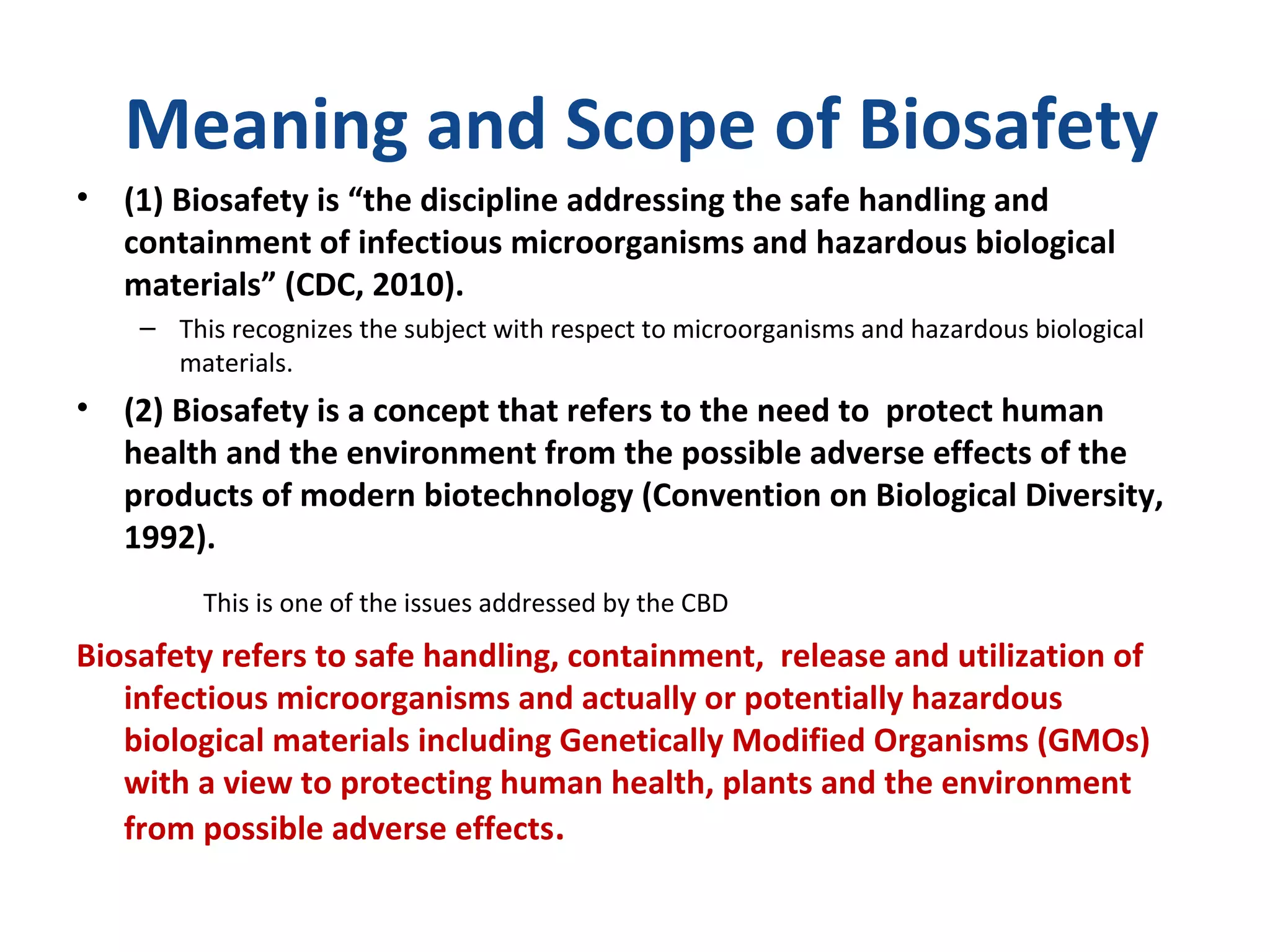
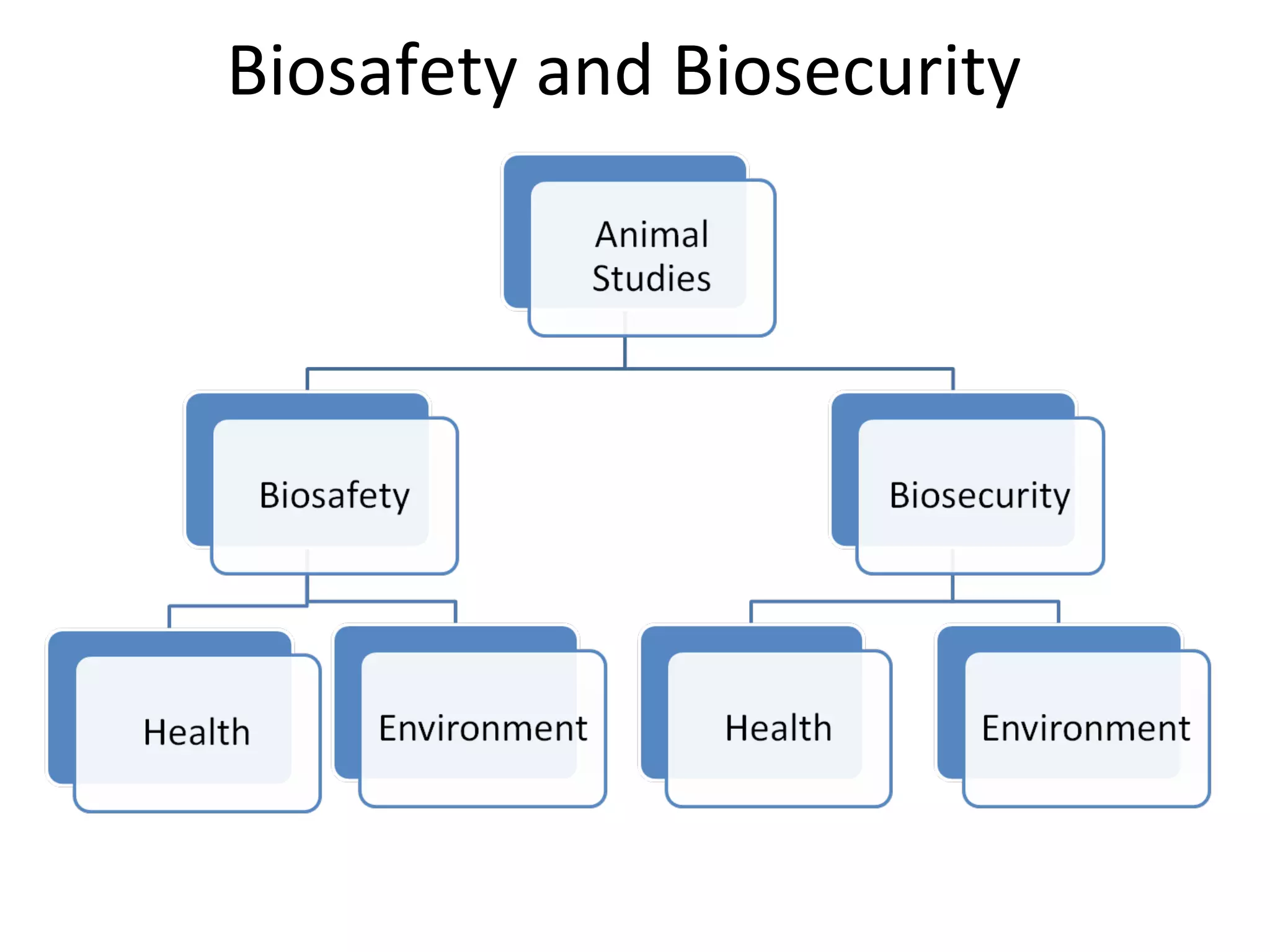
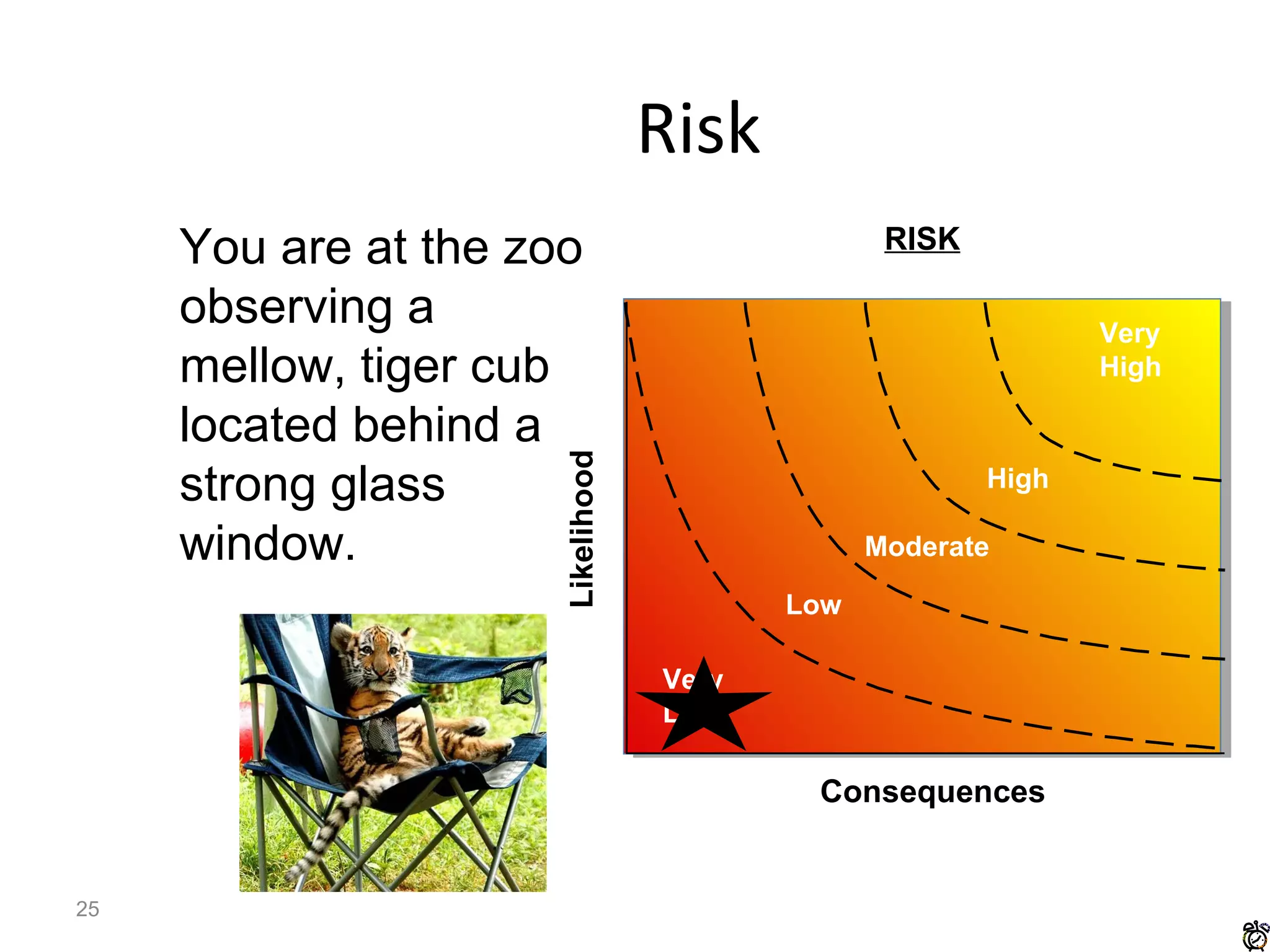
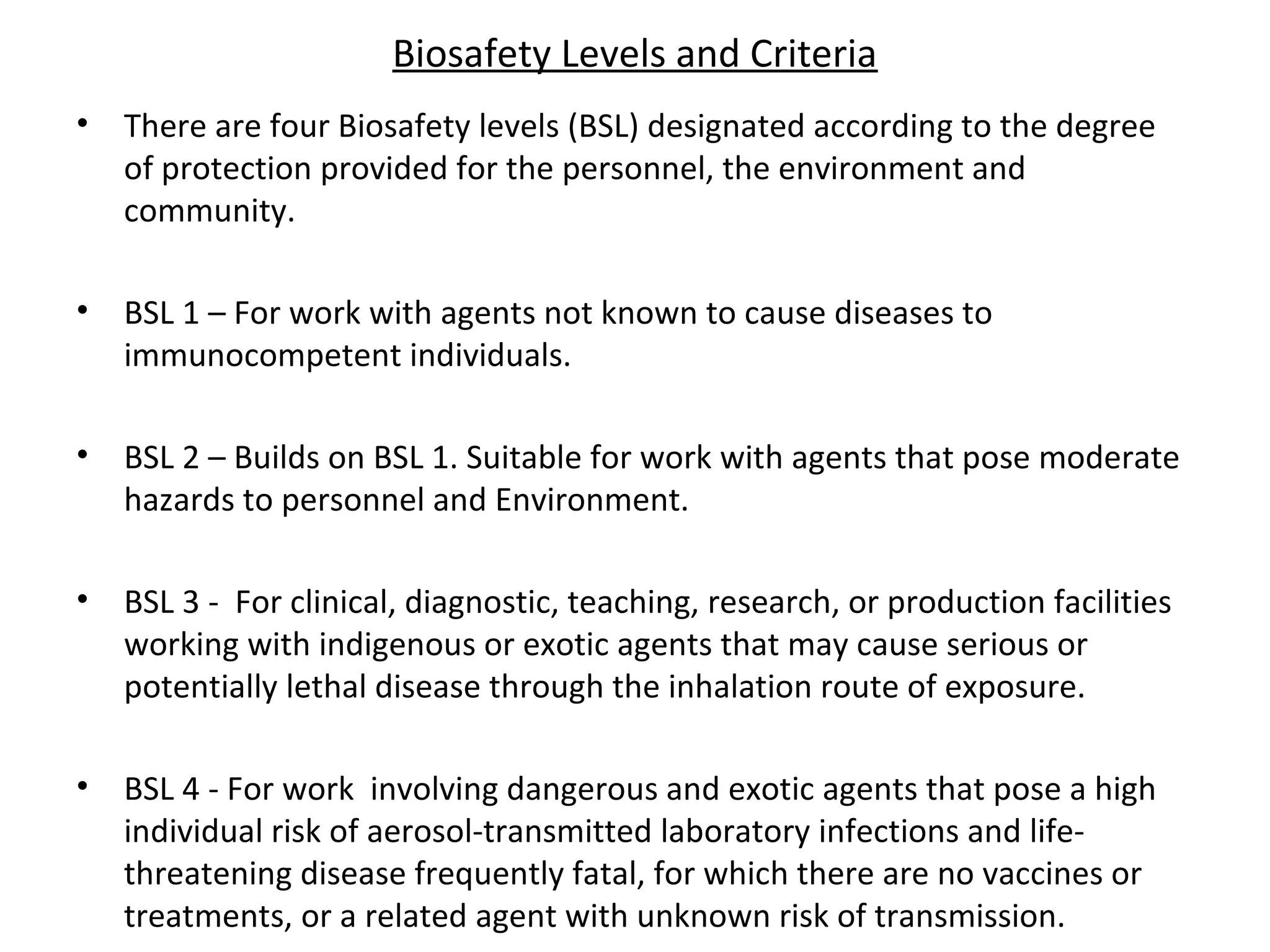
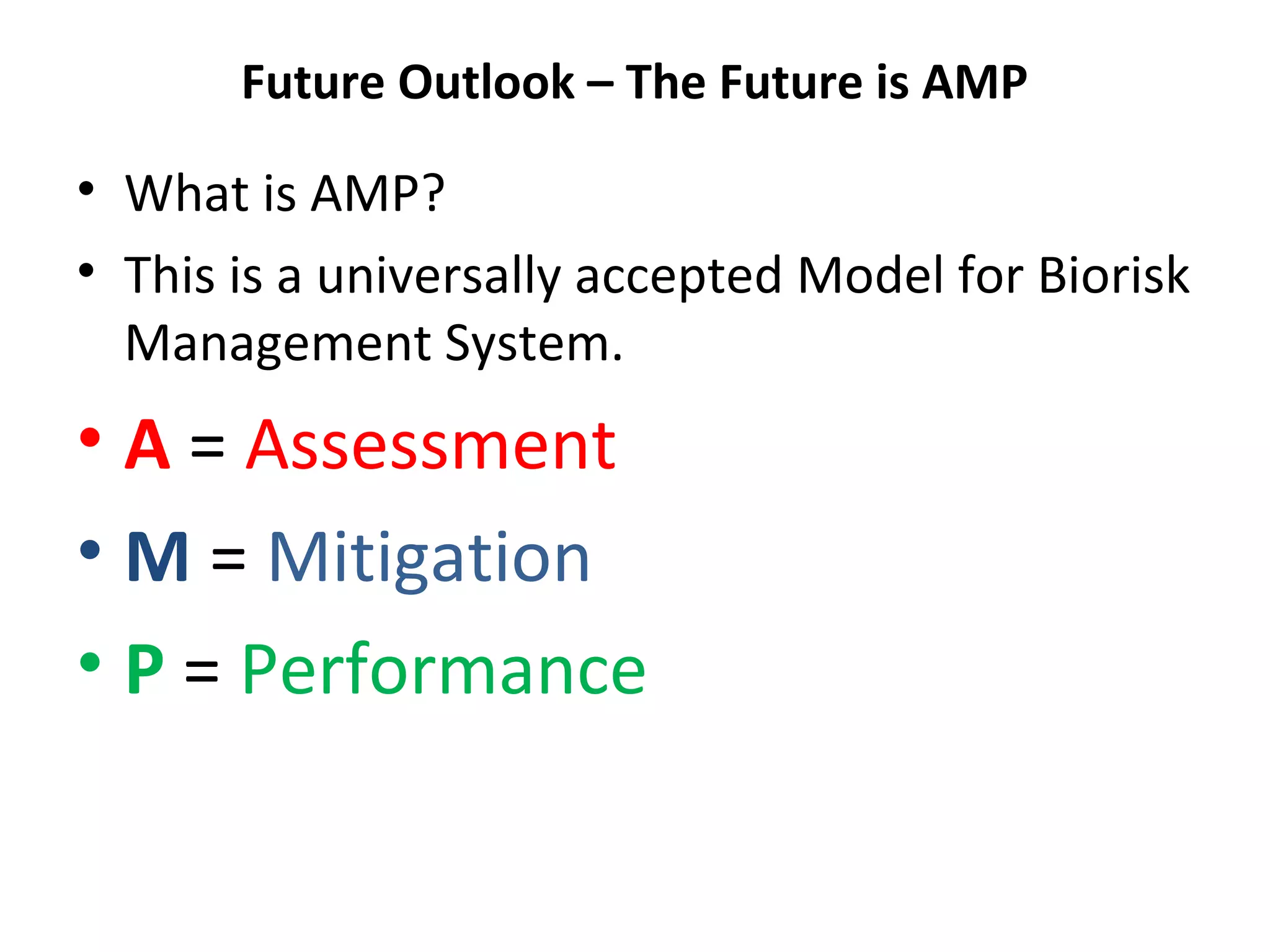
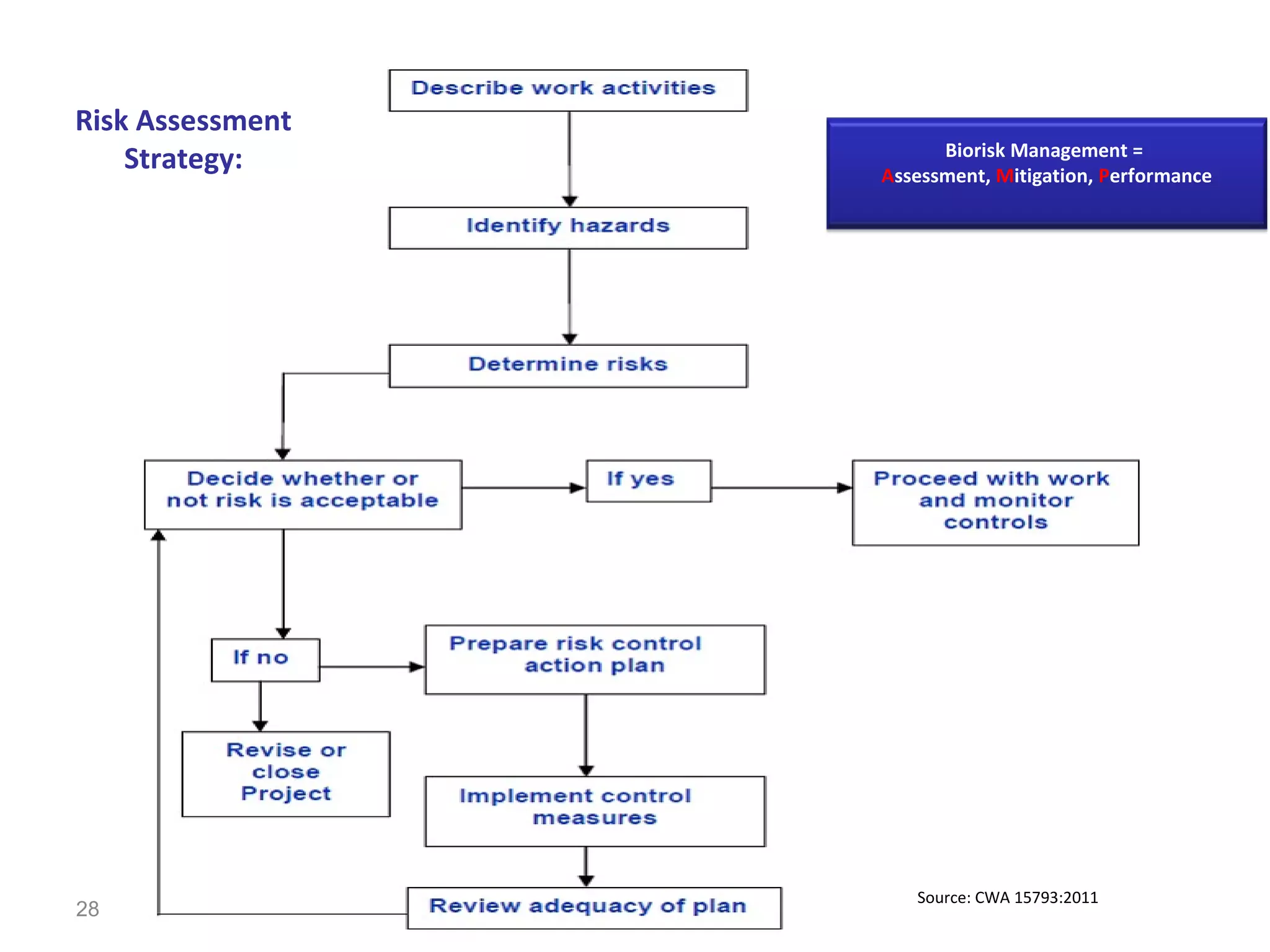
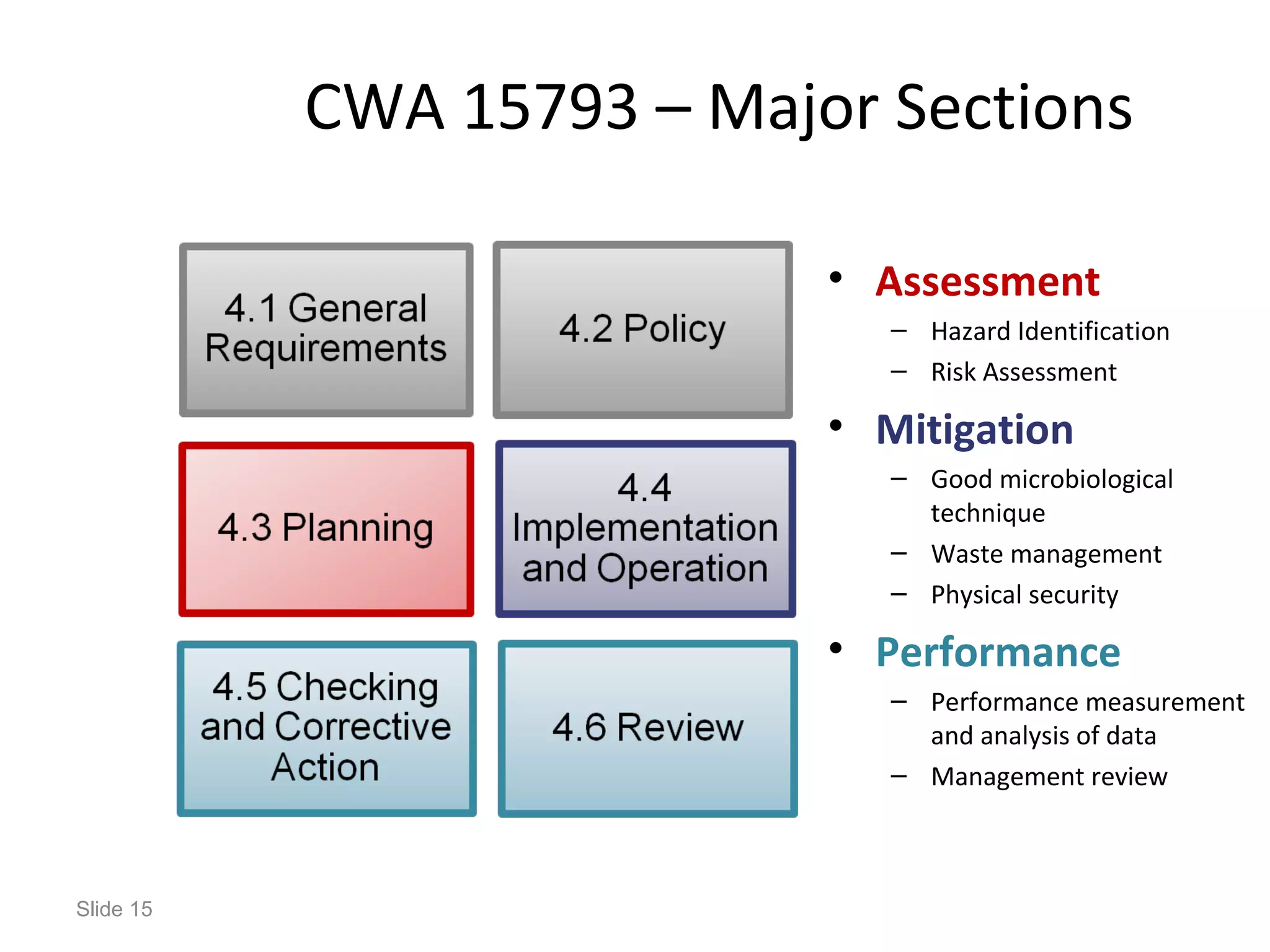
The document discusses biorisk management in relation to biosafety and biosecurity when working with animals in a laboratory setting. It outlines key concepts, risks associated with various biological materials, and biosafety levels 1 to 4, while emphasizing the need for effective management and compliance with international guidelines such as CWA 15793. It also highlights the roles and responsibilities of zoologists in ensuring safe and secure handling of biological agents in research and training.