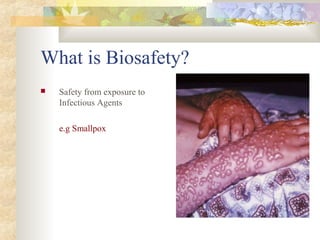
The document discusses biosafety concepts and practices. It begins by defining biosafety as safety from exposure to infectious agents. It then discusses biosafety issues in various disciplines like agriculture, medicine, and chemistry. The rest of the document outlines biosafety concepts, levels, and practices based on guidance from the Biosafety in Microbiological and Biomedical Laboratories (BMBL) including standard microbiological practices, safety equipment, and facility design requirements for different biosafety levels from BSL-1 to BSL-4. It also discusses risk assessment and containment practices for working with various biological hazards.