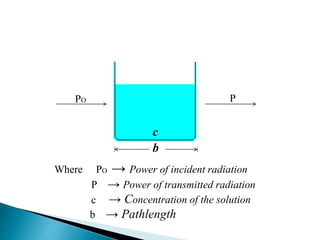
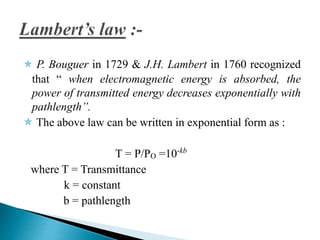
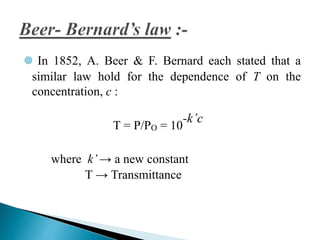
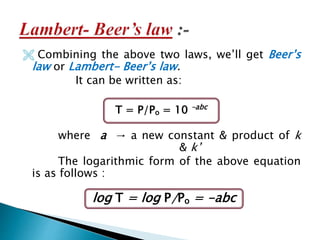
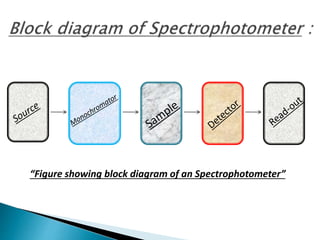
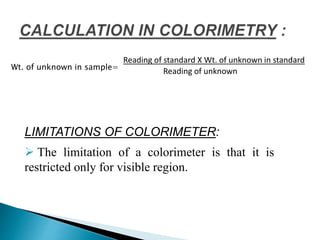
This document discusses spectrophotometry and colorimetry. It begins by introducing spectrophotometry, the basic principle of Beer's law, and applications of spectrophotometry such as structure elucidation and concentration calculations. It then discusses colorimetry, noting it is based on Beer's law and used to estimate the concentration of colored substances. The document outlines the basic principles, calculations, applications, and limitations of both spectrophotometry and colorimetry. It concludes by stating spectrophotometry is widely used for determination of complex structure, concentration, and reaction rates while colorimetry is restricted to visible light concentration determinations.