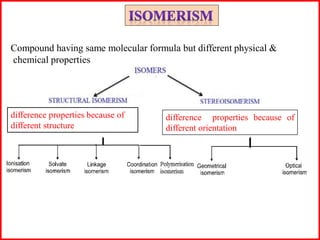
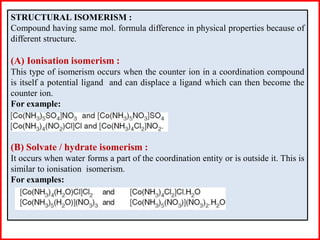
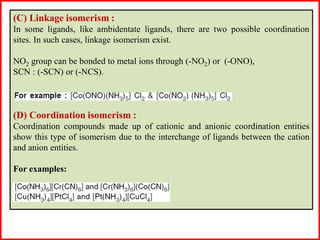
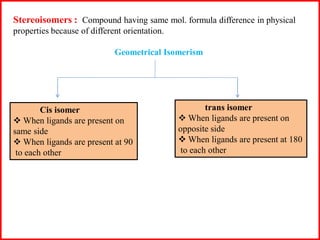
This document discusses different types of structural isomerism that can occur in coordination compounds. It defines structural isomerism as compounds having the same molecular formula but different physical and chemical properties due to different structures or orientations. The types of structural isomerism discussed include ionization isomerism, solvate/hydrate isomerism, linkage isomerism, coordination isomerism, ligand isomerism, polymerization isomerism, geometrical isomerism (cis/trans), and optical isomerism. Examples are provided to illustrate each type of isomerism.

![(E) Ligand isomerism :
Since many ligands are organic compounds which have possibilities for
isomerism, the resulting complexes can show isomerism from this source.
For example :
ligands 1,2-diaminopropane(propylenediamine or pn) and
1,3-diaminopropane(trimethylenediamine or tn)
(F) Polymerisation isomerism :
Considered to be a special case of coordination isomerism, in this the various
isomers differ in formula weight from one another
For example :
These all have the empirical formula [Pt(NH3)2Cl2] 1: 2 :2
[Pt(NH3)2Cl2] Pt:NH3:Cl 1: 2 :2
[Pt(NH3)4] [PtCl4] Pt:NH3:Cl 2: 4 :4](https://image.slidesharecdn.com/isomerism-201203094427/85/Isomerism-5-320.jpg)
![Coordination Number Four :
Tetrahedral Complex :
The tetrahedral compounds can not show geometrical isomerism as we all know
that all four positions are equivalent in tetrahedral geometry.
[MA4], [MA3B] not show G. I.
Square Planar Complex :
In a square planar complex of formula [MA2B2] [A and B are unidentate], the two
ligands .A. may be arranged adjacent to each other in a cis isomer, or opposite to
each other in a trans isomer as depicted.
[MA2B2], [M(AB)2], [MA2BC], [MABCD] show G. I.](https://image.slidesharecdn.com/isomerism-201203094427/85/Isomerism-7-320.jpg)
![Coordination Number 6 :
Geometrical isomerism is also possible in octahedral complexes.
[MA6], [MA5B], [M(AA)3] not show G. I.
[MA2B2C2], [MA2B2CD], [MA2BCE], [MABCDEF], [M(AB)3], [MA4B2], [MA3B3]
show G. I.
Optical Isomerism :
Plane of symmetry
Center of symmetry
Axis of symmetry
Should not be present](https://image.slidesharecdn.com/isomerism-201203094427/85/Isomerism-8-320.jpg)
![• Tetrahedral complexes with formula [MABCD] show optical isomers and
octahedral complexes (cis form) exhibit optical isomerism.
• Optical isomerism is common in octahedral complexes involving didentate
ligands. Cis-isomer of [PtCl2(en)2]2+ show optical isomerism as shown below
because of the absence of plane of symmetry as well as centre of symmetry.](https://image.slidesharecdn.com/isomerism-201203094427/85/Isomerism-9-320.jpg)
