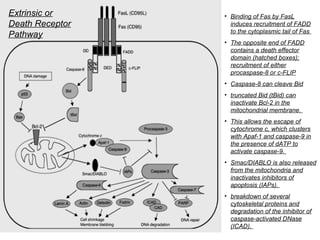
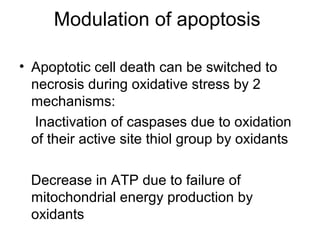
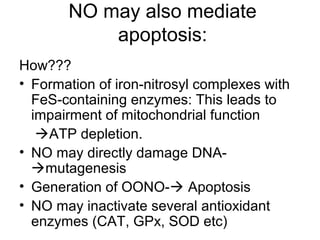
1) Apoptosis, or programmed cell death, is a natural and important process by which cells self-destruct in response to internal or external signals. It plays a key role in development, tissue homeostasis, and defense against infection and cancer.
2) There are two main pathways of apoptosis - the extrinsic or death receptor pathway, which involves cell surface death receptors, and the intrinsic or mitochondrial pathway, which is activated by intracellular stresses. Both pathways activate caspases, cysteine proteases that dismantle the cell in an orderly manner.
3) Apoptosis is tightly regulated by a network of pro- and anti-apoptotic Bcl-2 family proteins that control mitochondrial membrane permeability and