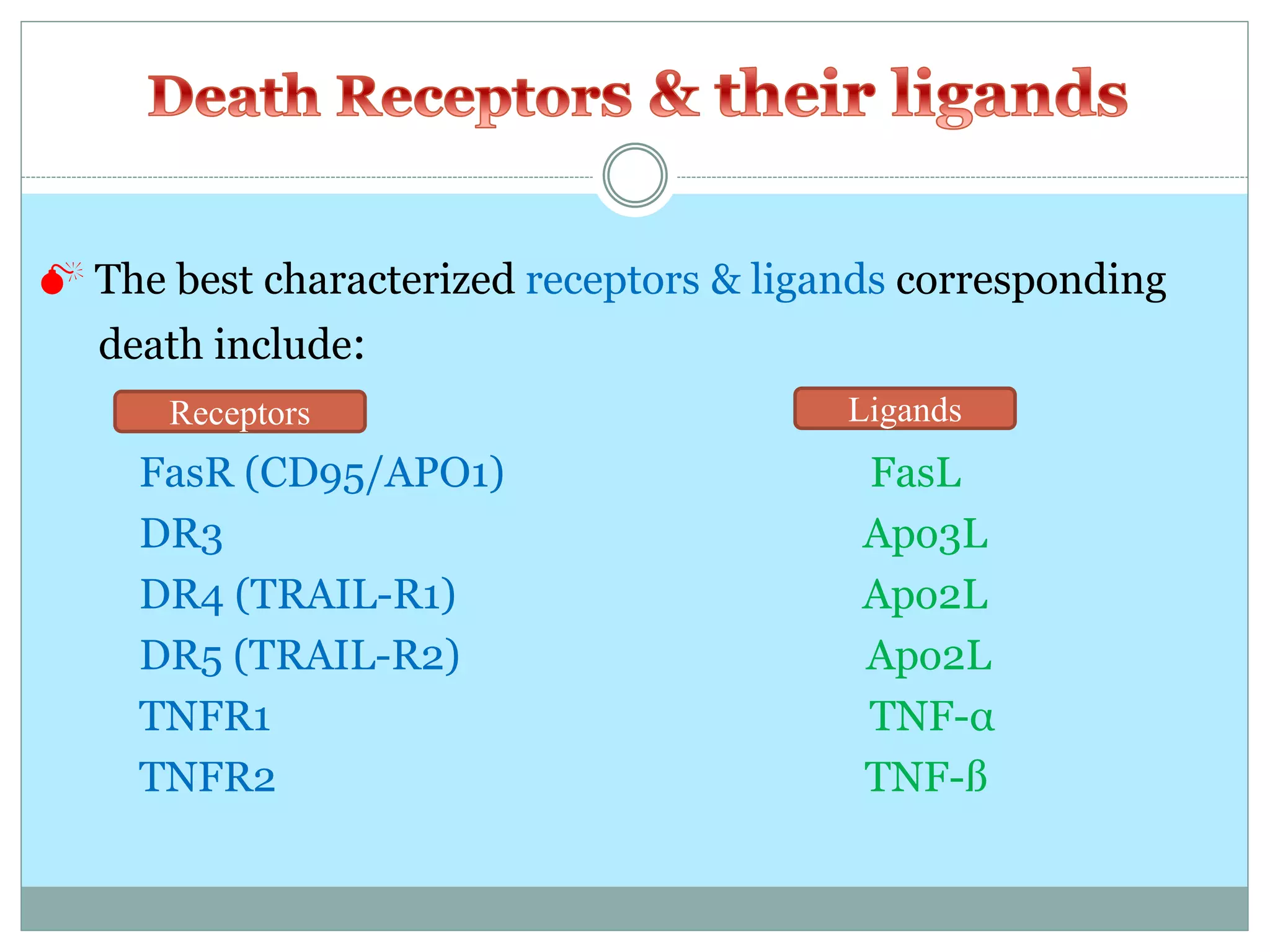
This document provides an overview of apoptosis, or programmed cell death. It begins by defining apoptosis and necrosis, explaining that apoptosis is normal and programmed cell death while necrosis is accidental cell death. It then discusses the importance of apoptosis in development and homeostasis. The key events and mechanisms of apoptosis are described, including the roles of caspases, Bcl-2 proteins, cytochrome c, death receptors/ligands, and the intrinsic and extrinsic pathways. Differences between apoptosis and necrosis are highlighted. The summary concludes by noting how aberrant cell death can lead to diseases like cancer or neurodegeneration.