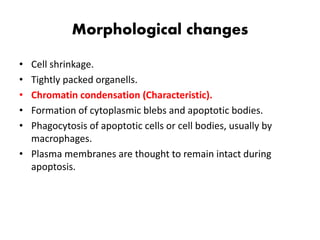
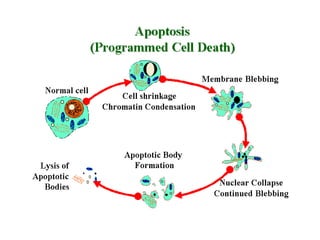
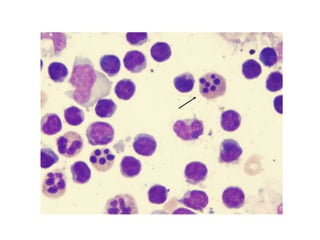
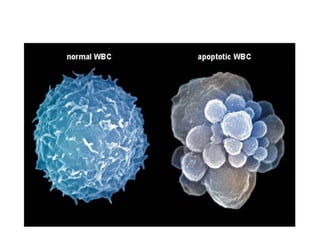
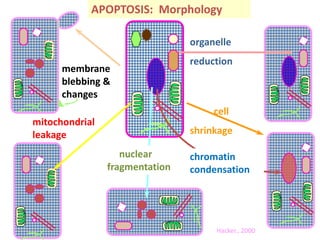
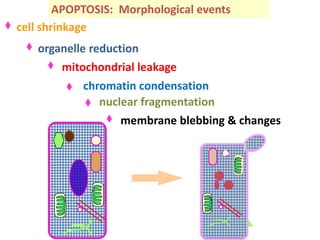
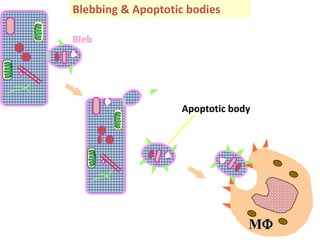
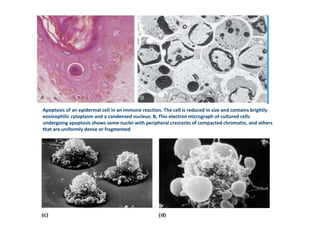
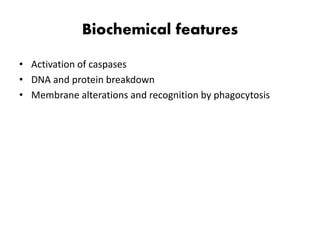
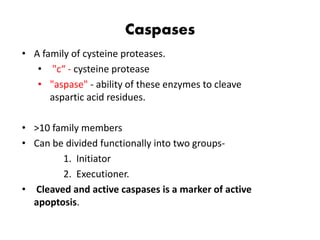
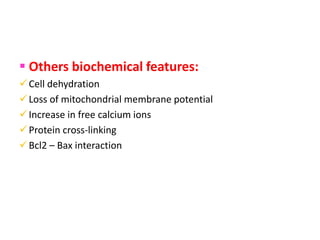
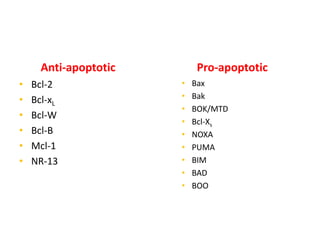
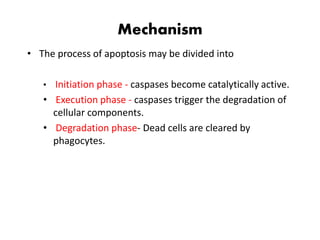
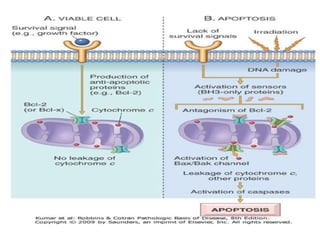
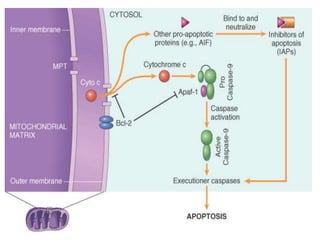
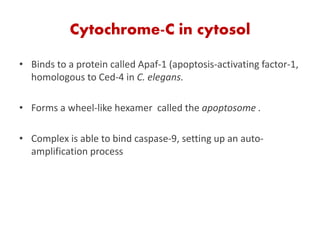
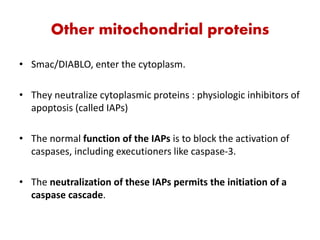
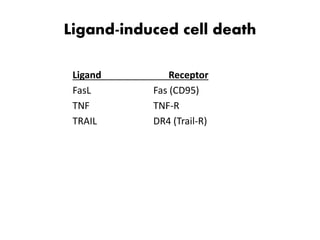
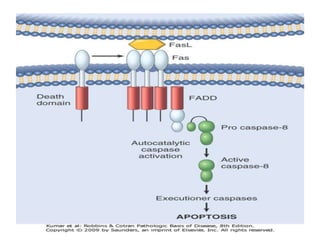
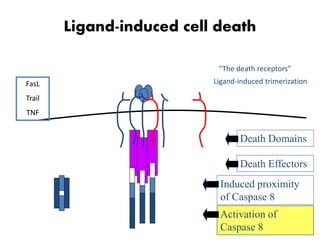
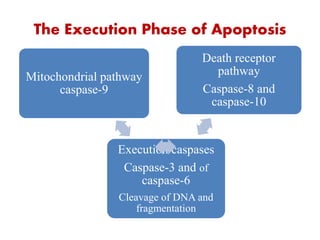
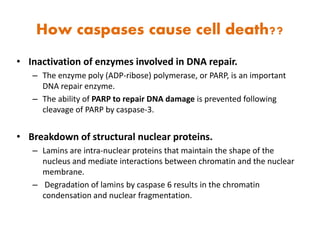
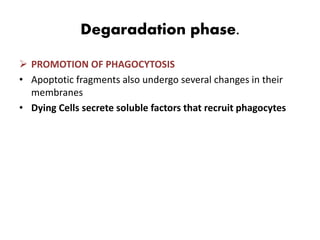
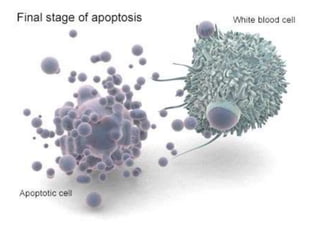
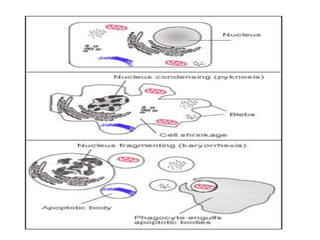
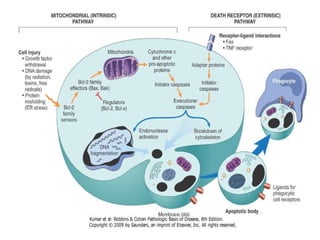
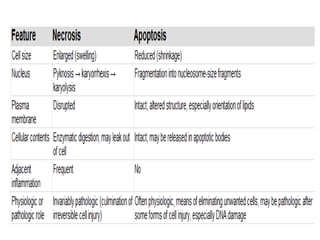
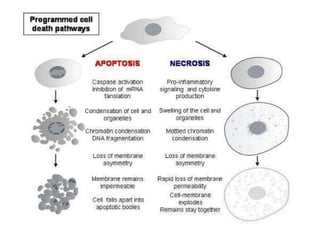
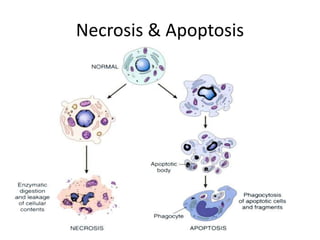
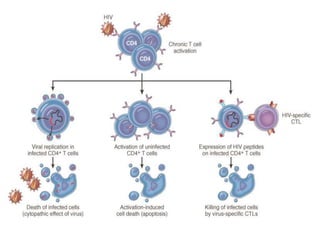
1. Apoptosis is a tightly regulated process of programmed cell death that involves the activation of caspases and degradation of nuclear and cellular components.
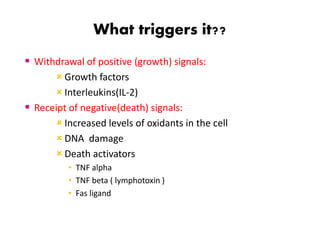
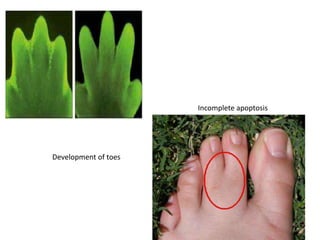
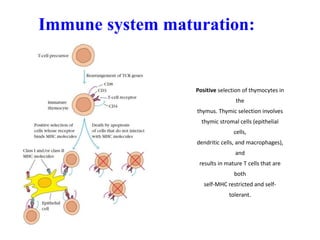
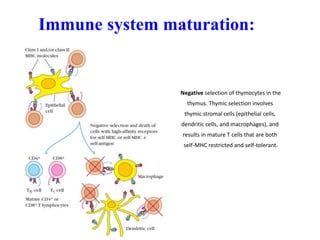
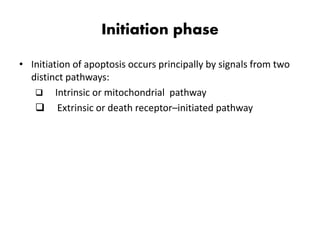
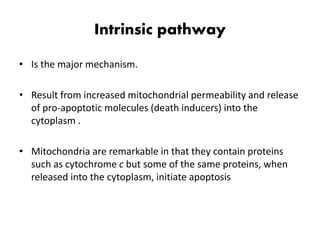
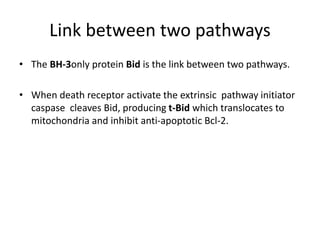
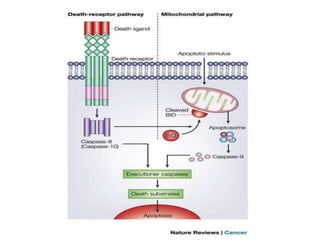
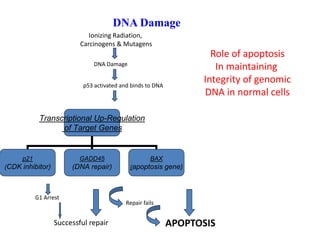
2. It can be triggered through intrinsic mitochondrial pathways or extrinsic death receptor pathways and plays an important physiological role in development, immune system maturation, and maintenance of tissue homeostasis.
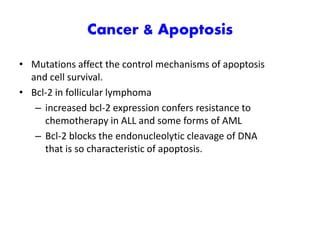
3. Dysregulation of apoptosis can contribute to cancer, autoimmune diseases, and neurodegenerative disorders by allowing cells to survive inappropriately or undergo excessive cell death.