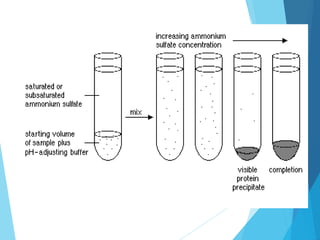
Ammonium sulphate precipitation is a technique that exploits differences in protein solubility. As ammonium sulphate concentration increases, water molecules hydrate salt ions instead of protein surfaces. This exposes hydrophobic protein regions, causing aggregation and precipitation. Proteins precipitate over a narrow range of salt concentrations, allowing selective enrichment. A sample can be fractionated by successive precipitation steps at increasing ammonium sulphate percentages, isolating proteins based on their solubility thresholds.