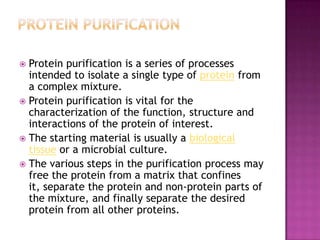
Protein purification involves a series of steps to isolate a single protein from a complex mixture. These steps may separate proteins based on size, charge, or binding affinity. Common techniques include precipitation with ammonium sulfate, chromatography methods like ion exchange, affinity, size exclusion, and reversed-phase chromatography, and electrophoresis. The goal is to free the protein of interest from other materials, separate it from other proteins, and finally isolate it in a pure form for characterization and use.