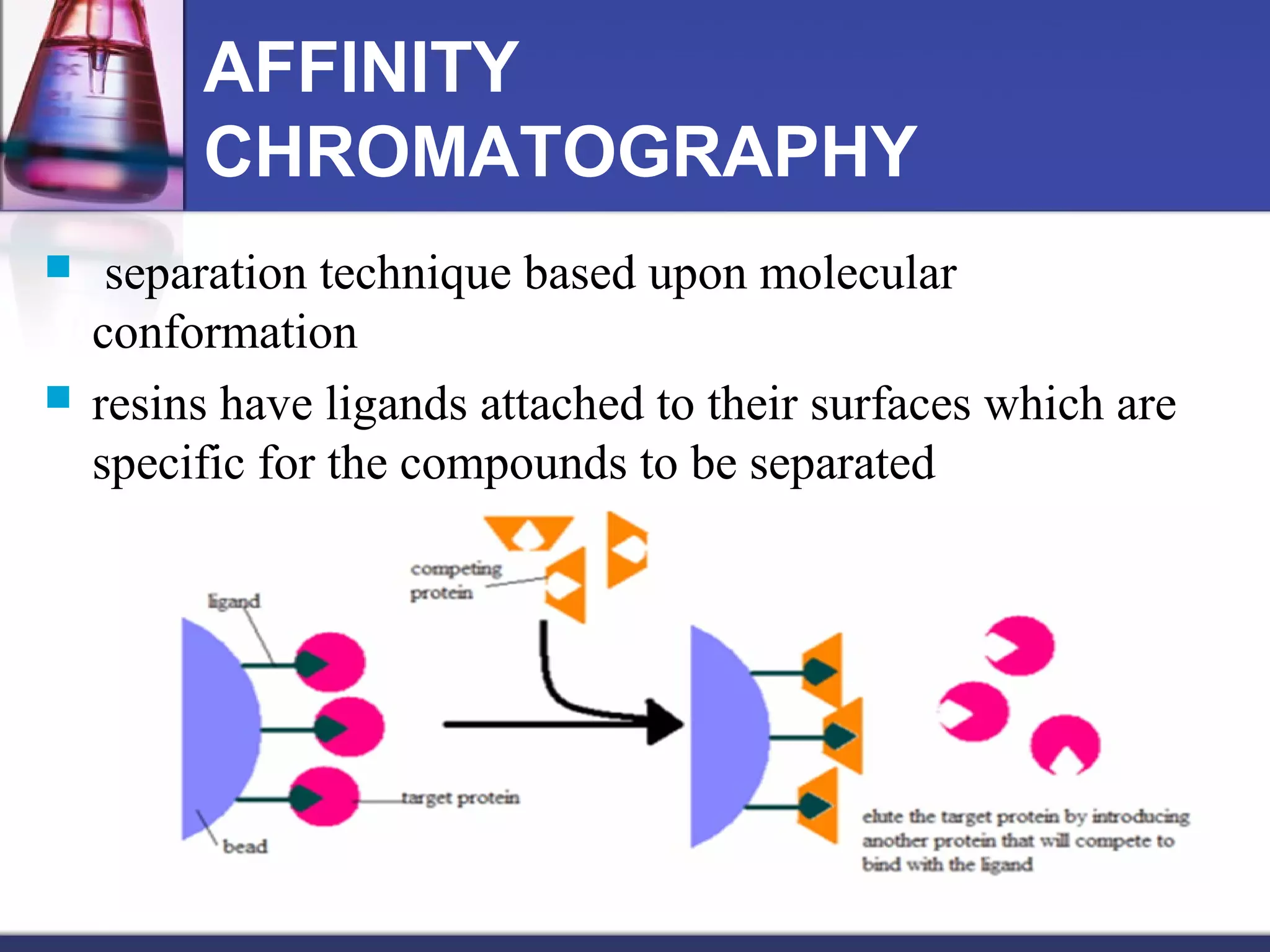
Protein purification techniques involve isolating a single protein from a complex mixture in order to characterize its function, structure, and interactions. The main steps include extracting proteins from cells, stabilizing them in solution, separating them using techniques like precipitation, filtration, dialysis, and various types of chromatography, and then verifying the purity of the isolated protein using analytical methods like SDS-PAGE and mass spectrometry. The basis of protein separation includes differences in solubility, binding interactions, size, charge, and other properties.