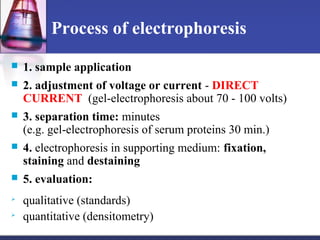
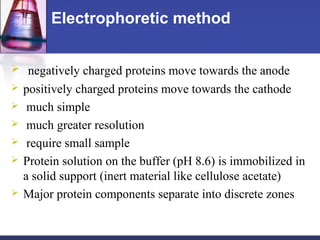
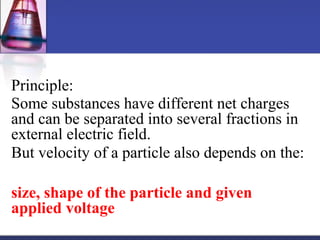
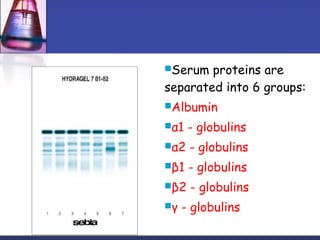
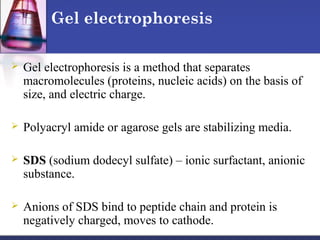
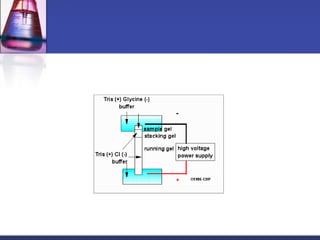
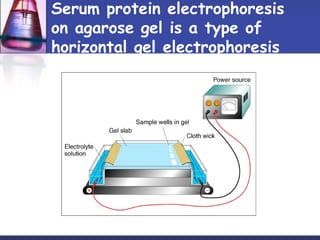
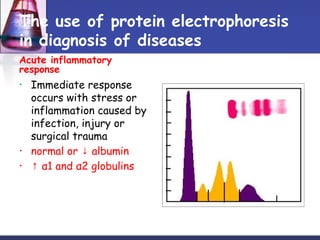
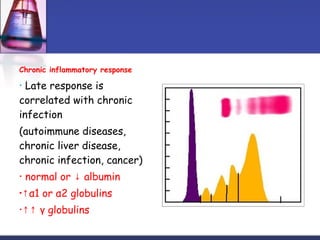
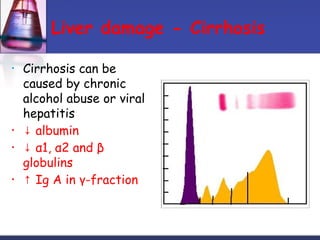
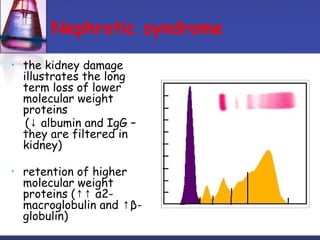
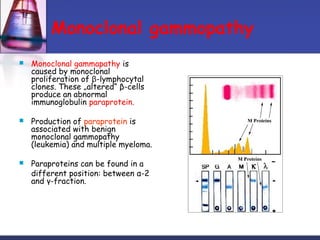
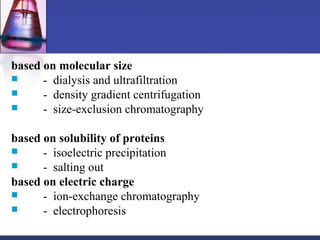
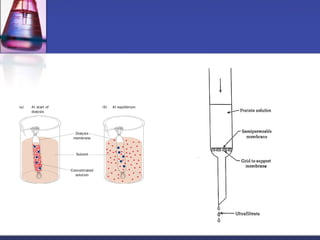
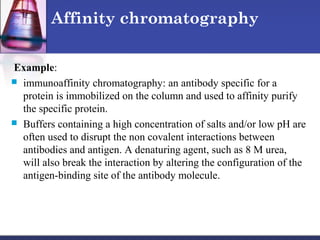
Protein purification techniques can be categorized into those based on molecular size, solubility, and electric charge. Size-based techniques include dialysis, ultrafiltration, and size-exclusion chromatography which separate proteins based on their ability to pass through semi-permeable membranes or porous beads. Solubility-based techniques include isoelectric precipitation and salting out which alter a protein's solubility by adjusting pH or salt concentration. Charge-based techniques such as ion-exchange and electrophoresis separate proteins using their net electric charge in an applied electric field or ion-exchange column.


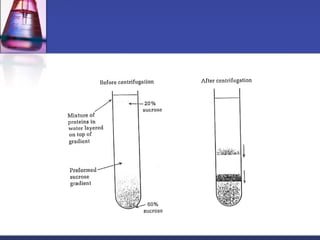
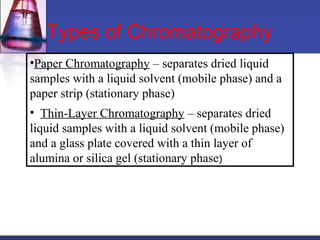
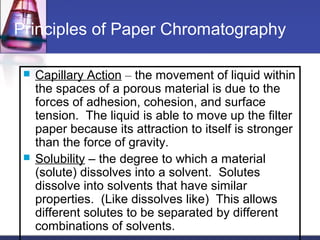
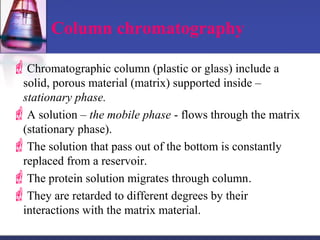
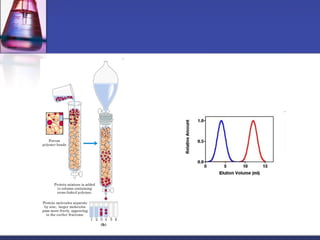
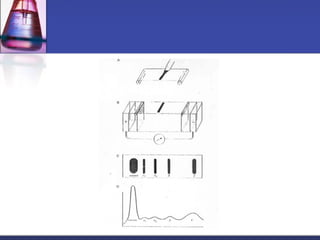
![Gel-filtration chromatography
Method uses porous particles to separate molecules of different
size.
proteins passed over a column filled with a hydrated porous
beads made of a carbohydrate or polyacrylamide polymer.
mixture of proteins dissolved in suitable buffer, is allowed to flow
by gravity down a column.
column is packed with beads of inert polymeric material.
very large molecules cannot penetrate into the pores of the beads,
the small molecules enter the pores.
large molecules are excluded and small proteins are retarded
[large molecules exit (elute) first] .](https://image.slidesharecdn.com/purificationtechniques-140609085810-phpapp02/85/Purification-techniques-14-320.jpg)
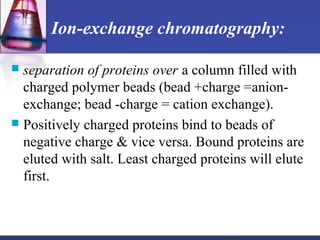
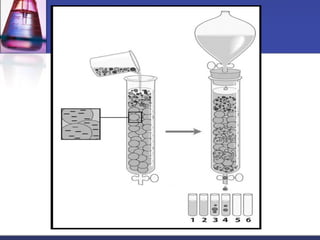
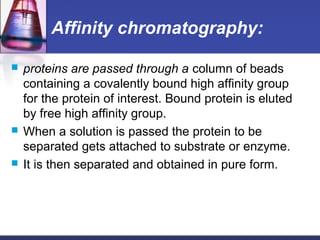


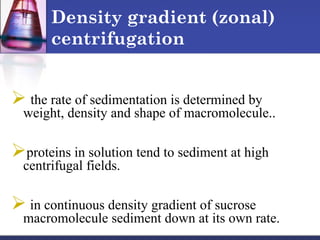
![ In general:
a) small proteins more soluble than large proteins
b) the larger the number of charged side chains, the more
soluble the protein
c) Proteins are usually least soluble at their isoelectric
points.
Sufficiently high ionic strength completely precipitate a
protein from solution.
Divalent salts [MgCl2, (NH4)SO4] are far more effective
than monovalent (NaCl).](https://image.slidesharecdn.com/purificationtechniques-140609085810-phpapp02/85/Purification-techniques-23-320.jpg)