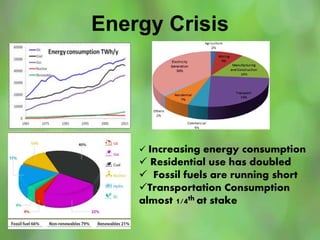
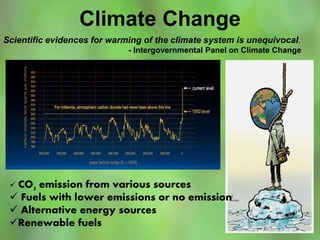
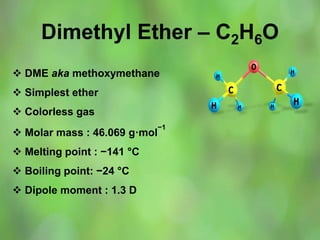
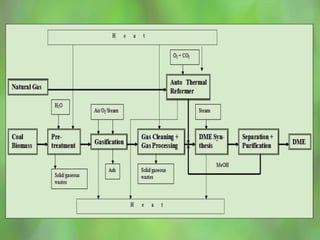
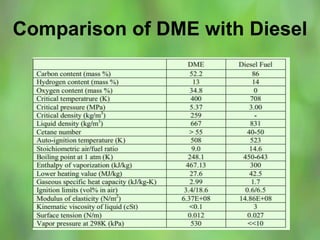
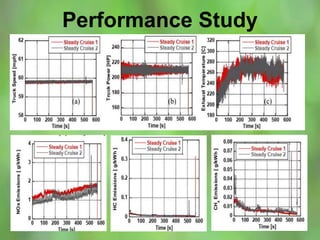
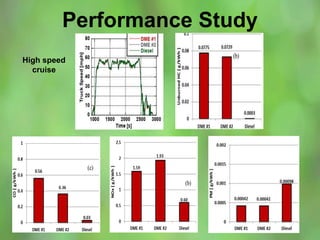
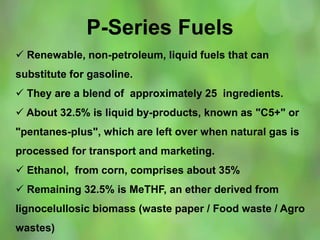
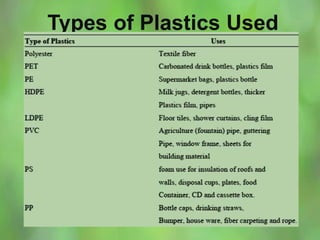
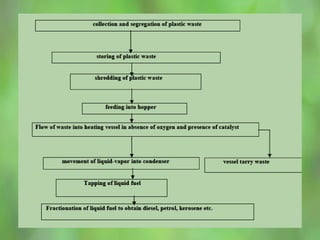
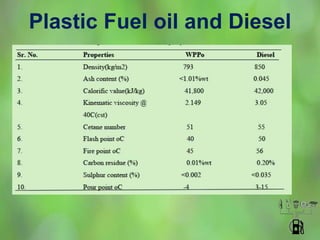
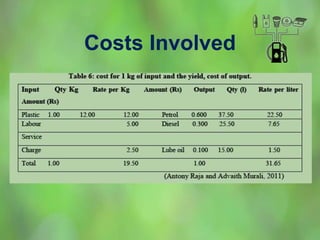
This document discusses alternate synthetic fuels as alternatives to traditional fossil fuels. It covers several types of synthetic fuels including dimethyl ether (DME) and plastic-derived fuels. DME can be produced from biomass, methanol, or natural gas, and has benefits over diesel like lower emissions but challenges like higher vapor pressure. Plastic-derived fuels involve pyrolyzing plastic waste at high temperatures to produce synthetic crude or refined fuels without combustion. This process can help address the global plastic waste problem while producing a usable fuel.