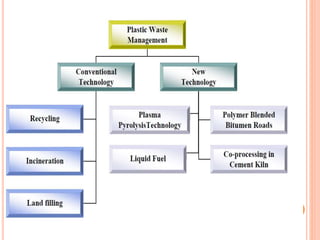
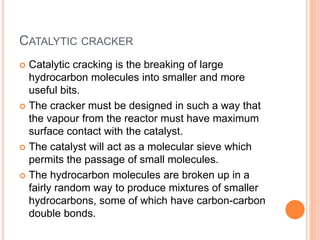
The document discusses the conversion of waste plastics into fuel through the process of pyrolysis, which involves thermal degradation in an oxygen-free environment. It outlines the benefits and technologies used, including the production of solid, liquid, and gaseous fuels with reduced sulfur content. Additionally, it highlights the operational components of pyrolysis machines and emphasizes the environmental advantages of using this method to manage plastic waste.