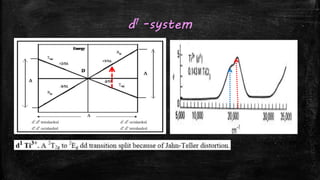
This document discusses using Orgel and Tanabe-Sugano diagrams to calculate crystal field splitting energies (10 Dq) and Racah parameters (B) for various d-block metal complexes. It provides examples of calculating these values for d1, d2, d7, and d9 systems using observed absorption bands and relationships between the transitions. The values obtained are then used to determine 10 Dq, B, and the nephelauxetic ratio β for complexes such as [Ti(H2O)6]3+, [V(H2O)6]3+, and [Co(H2O)6]2+.



![▪ Consider the example of [Ti(H2O)6]3+
▪ Calculation of B: No need to calculate the Racah parameter since
there is only one electron.
▪ Calculation of Δo: The purple color of the complex ion [Ti(H2O)6]3+
is due to a broad absorption band at 20300 cm-1 arising from 2T2g →
2Eg transition.
▪ Hence, 10 Dq for this complex is 20300 cm-1.
▪ Calculation of β: No need to calculate the nephelauxetic ratio, since
only one electron is there.](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-6-320.jpg)


![▪ Consider the example of [Cu(H2O)6]2+
▪ Calculation of B: No need to calculate the Racah parameter
▪ Calculation of Δo: In the UV-visible spectra of [Cu(H2O)6]2+the
broad band at 12000 cm-1
▪ This is due to spin allowed 2Eg → 2T2g transition; and hence,
10 Dq for this complex is 12000 cm-1.
▪ Calculation of β: No need to calculate the nephelauxetic ratio.](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-9-320.jpg)
![▪ Metal complexes with d2-configuration have 3F ground state
term symbol in the absence of any crystal field.
▪ However, when six ligands approach in octahedral coordination,
the ground state term symbol becomes 3T1g and remains as
such in weak as well as in strong ligand fields.
▪ The Orgel and Tanabe-Sugano diagram for d2-configuration can
be used to estimate the value of crystal field splitting energy
for these transition metal complexes.
Example:
[V(H2O)6]3+
V3+ contains two d electrons](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-11-320.jpg)

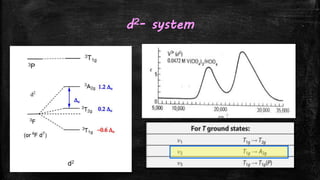
![d2-system
▪ For a d2 system, [V(H2O)6]3+ is an ideal example.
▪ Two bands are observed.
▪ Third one may be too energetic, obscured by the CT bands.
▪ The first band is assigned easily to 3T1g 3T2g which is at 17800 cm-1
▪ If there is no term interaction i.e. x=0, then
ν1
3T1g 3T2 g = 8Dq+x =17800 - 8Dq = 17800
Dq = 2225 cm-1
▪ Wkt ν2 = 18Dq + x = 18Dq = 39600 cm-1
▪ So the other band is appearing at 25700 cm-1 cannot be ν2 it should be ν3](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-15-320.jpg)
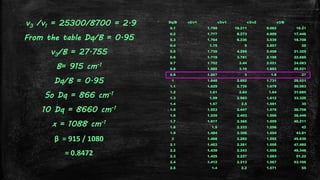
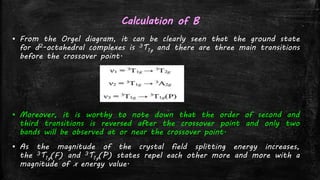
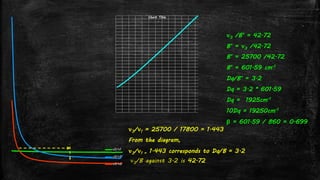
![Using Tanabe-Sugano diagram for d2 system – Lever’s Method
▪ From the Tanabe-Sugano diagram, in the UV-visible spectra of
[V(H2O)6]3+, two bands are observed with maxima at around
17800 and 25700 cm-1.
▪ There are three possible transitions expected, which include:
– ν1 = 3T1g→ 3T2g,
– ν2 = 3T1g → 3T1g(P)
– ν3 = 3T1g → 3A2g
– but only two are observed , since ν2 is obscured by CT bands.
▪ Now taking the ratio
ν3/ ν1 = 25700 / 17800 = 1.44](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-16-320.jpg)
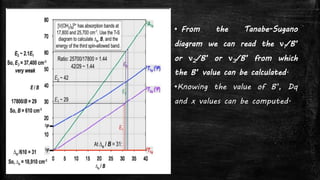
![d7- system
▪ Example: [Co(H2O)6]2+
▪ Ground term 4T1g
▪ Expected transitions three
– 4T1g 4T2g v1 = 8000 cm-1
– 4T1g 4A2g v2 = 19600 cm-1
– 4T1g 4T1g (P) v3 = 21600 cm-1](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-20-320.jpg)
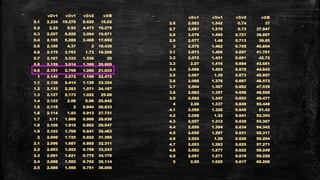
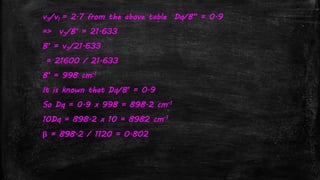
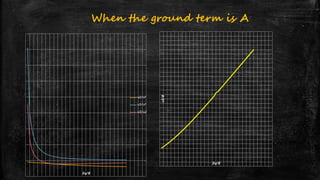

![Systems with A2 ground state- d3 system
▪ Example: [Cr(H2O)6]3+
▪ Ground term is 4A2g
▪ Three transitions expected
– 4A2g 4T2g (v1) = 10Dq
– 4A2g 4T1g (F) (v2) = 18Dq-x
– 4A2g 4T1g (P) (v3) = 12Dq+15B’+x
▪ v1 occurs at 17,400 cm-1
▪ v2 occurs at 24,600 cm-1
▪ v3 occurs at 37,800 cm-1](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-25-320.jpg)

![d8 system – [Ni(H2O)6]2+
•Example: [Ni(H2O)6]2+
•Ground term is 3A2g
• Three transitions are observed
3A2g 3T2g (v1) = 10Dq
3A2g 3T1g (F) (v2) = 18Dq-x
3A2g 3T1g (P) (v3) = 12Dq+15B’+x
v1 occurs at 8700cm-1
v2 occurs at 14500 cm-1
v3 occurs at 25300 cm-1](https://image.slidesharecdn.com/electronicspectraproblems-201005014556/85/Electronic-spectra-problems-27-320.jpg)