This document contains information about several chemistry topics:
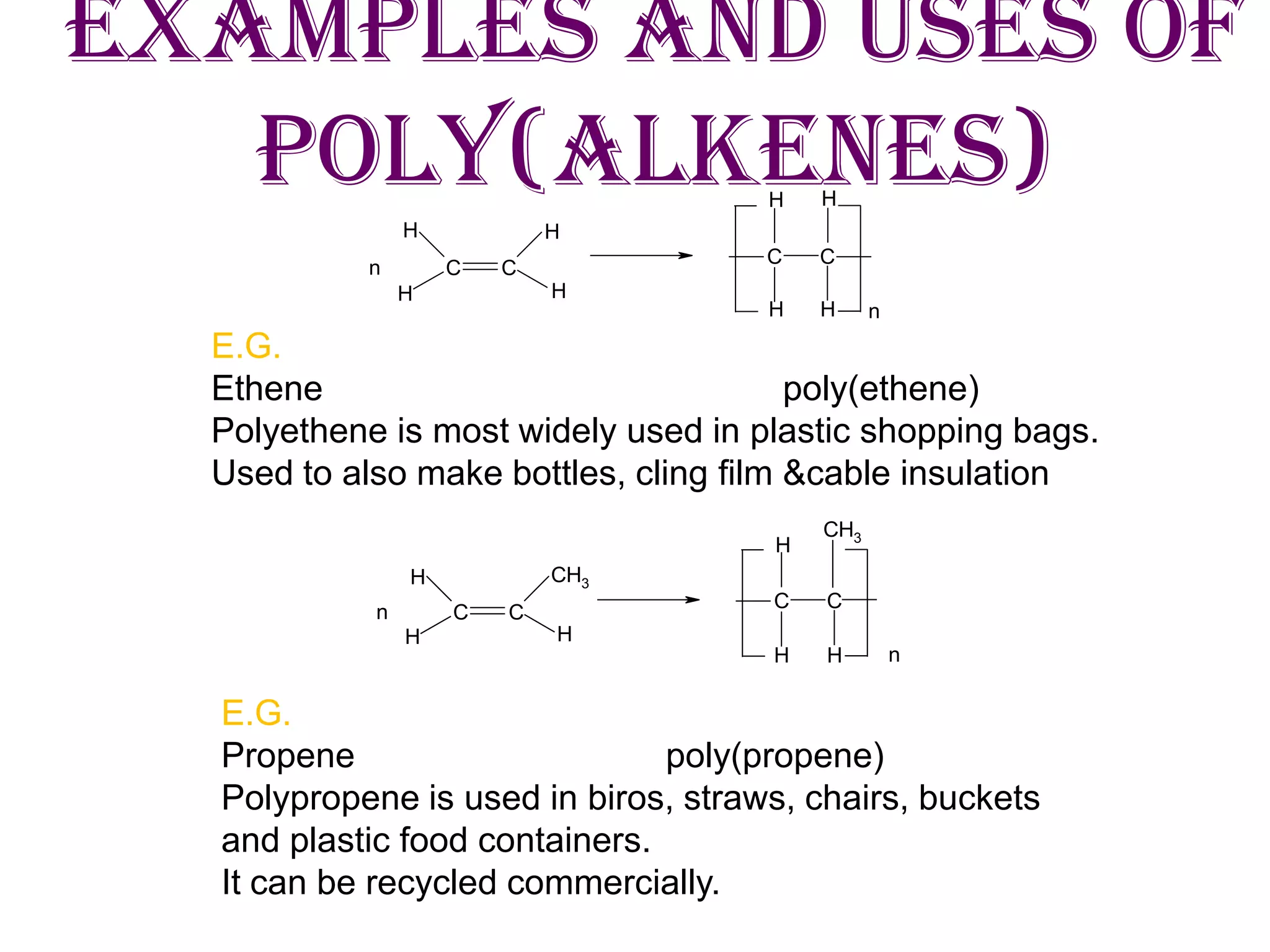
1) Polymerization of alkenes to form polymers like polyethene and polypropene which are used to make plastic bags, bottles, and other products.
2) Ethanol production through fermentation of sugars and its use as a biofuel.
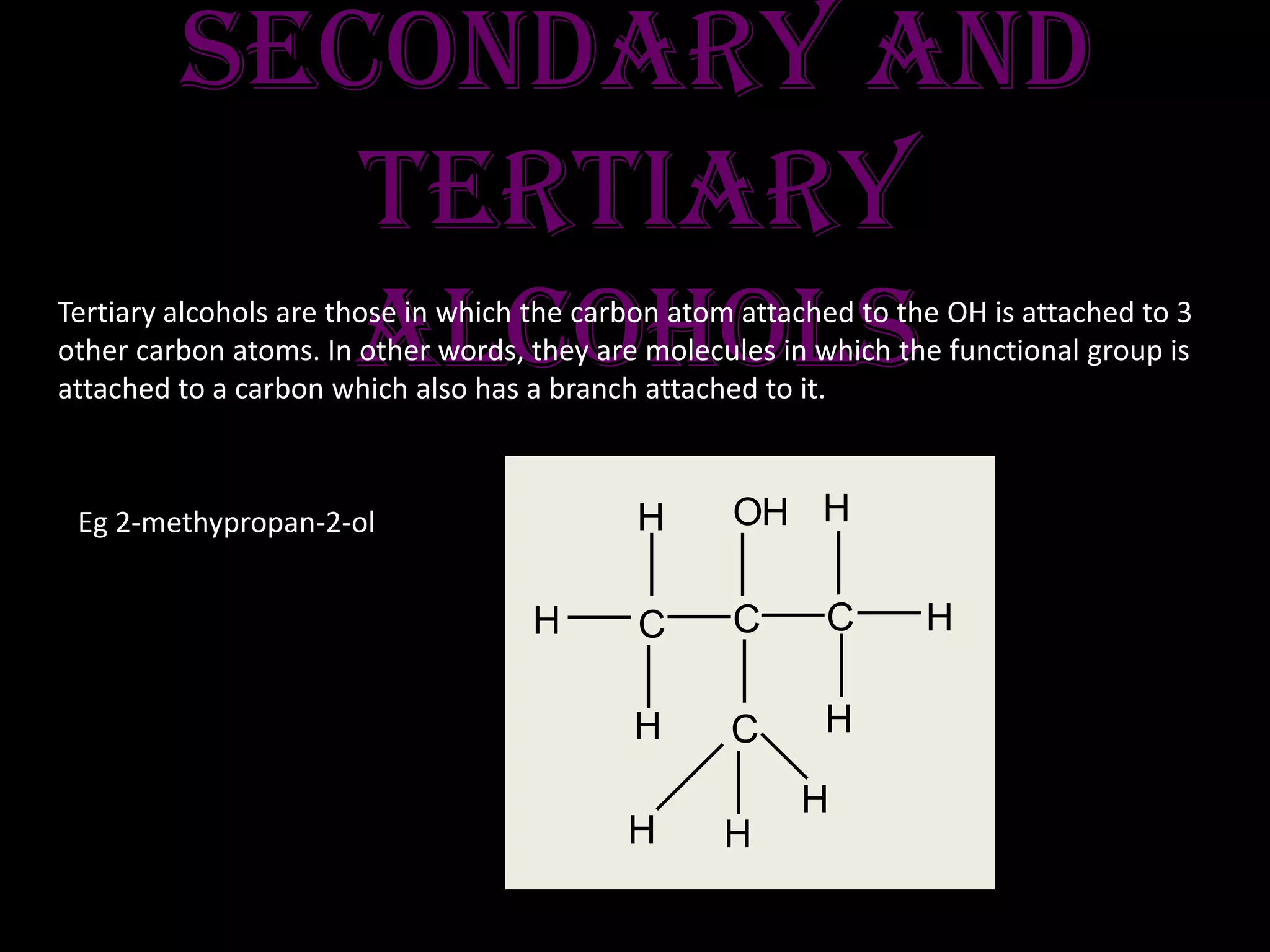
3) Classification of primary, secondary, and tertiary alcohols and their oxidation reactions to form aldehydes, ketones, or carboxylic acids depending on alcohol type.
4) Tests to distinguish between aldehydes and ketones using Tollen's reagent or Fehling's solution.








![H H
HH
CC OHH
+ [O]
+ H2OC
O
H
H
H
CH
C
O
H
C
O
CH3
CH3
CH
OH
CH3
CH3
+ [O] + H2O
C
O
If a primary alcohol is mixed with an oxidising agent, two hydrogen atoms can be removed and an aldehyde will be formed:
Eg CH3CH2OH + [O] CH3CHO + H2O
Ethanol Ethanal
An aldehyde is a molecule containing the following group:
If a secondary alcohol is mixed with an oxidising agent, two hydrogen atoms can be removed and a ketone will be formed:
Eg CH3CH(OH)CH3 + [O] CH3COCH3 + H2O
Propan-2-ol propanone
A ketone is a molecule containing the following group:
mild oxidation of primary and
secondary alcohols](https://image.slidesharecdn.com/ethanolproduction-130525160056-phpapp02/75/Ethanol-production-11-2048.jpg)
![Tertiary alcohols are not readily oxidised since they do not have available H atoms to give up.
Aldehydes and ketones are collectively known as carbonyls and can be represented by the general formula CnH2nO
C
O
R
R
In aldehydes, one of the R groups is a H atom. In ketones, neither of the R groups is a H atom.
Further oxidation of aldehydes
If an aldehyde is mixed with an oxidising agent, an oxygen atom can be added to the group and a carboxylic
acid will be formed:
Eg CH3CHO + [O] CH3COOH
Ethanal Ethanoic acid
A carboxylic acid is a molecule containing the following group:
Ketones cannot be oxidised into carboxylic acids since there is no C-H bond into which an oxygen
atom can be inserted.](https://image.slidesharecdn.com/ethanolproduction-130525160056-phpapp02/75/Ethanol-production-12-2048.jpg)
![Summary of oxidation
reactions of alcohols
and carbonylsprimary
alcohols
secondary
alcohols
tertiary
alcohols
aldehydes ketones
carboxylic
acids
X
[O]
[O]
[O]](https://image.slidesharecdn.com/ethanolproduction-130525160056-phpapp02/75/Ethanol-production-13-2048.jpg)

