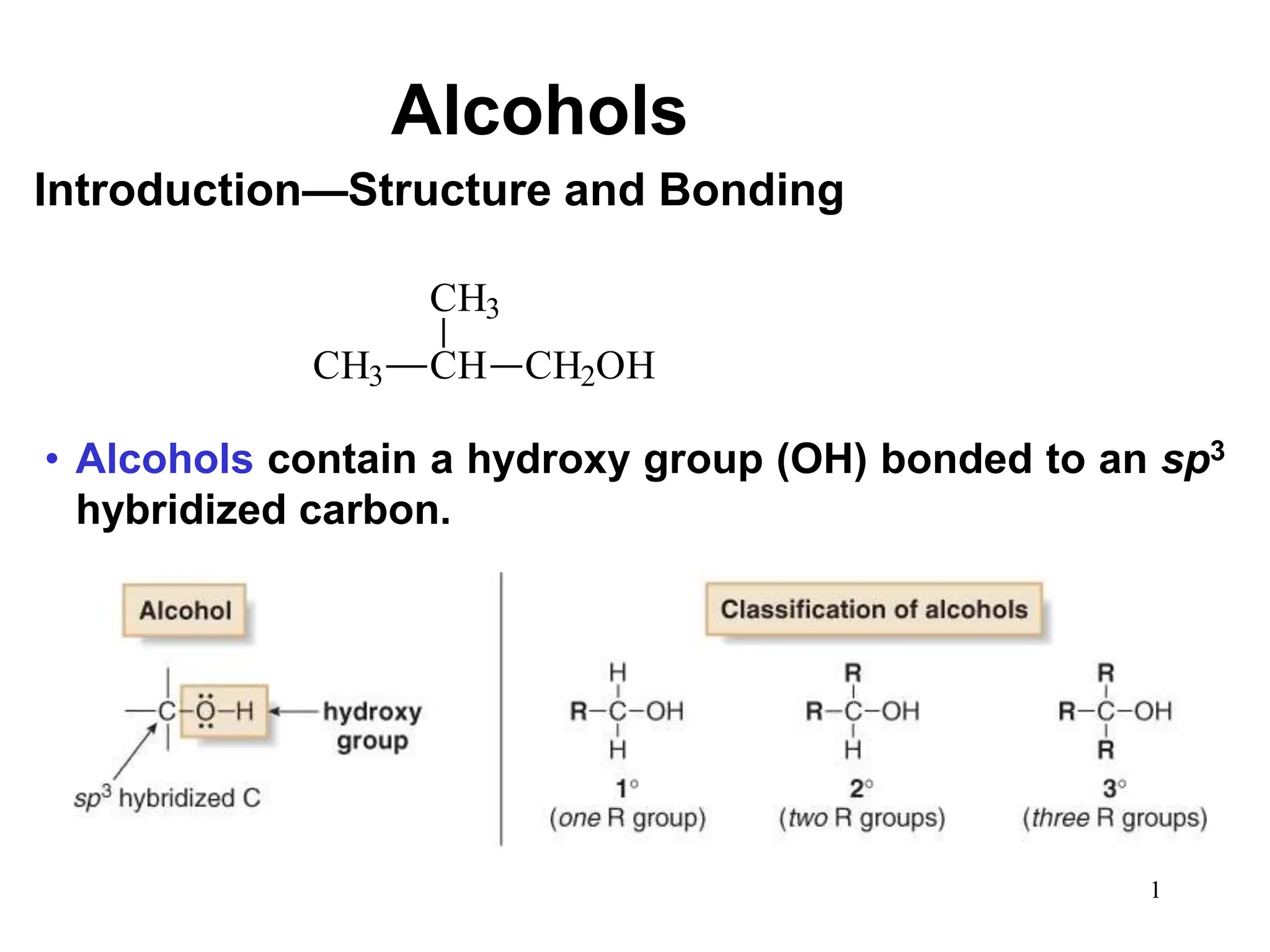
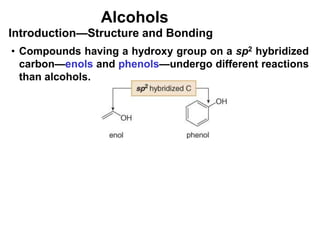
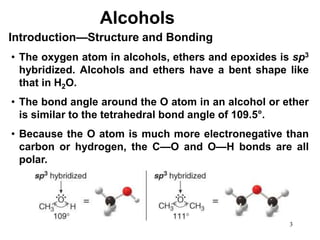
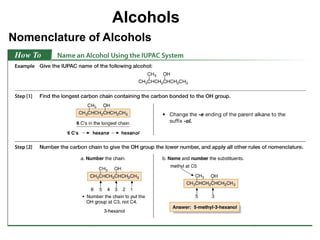
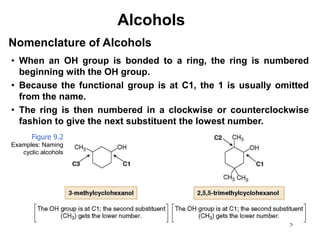
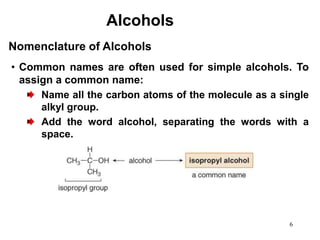
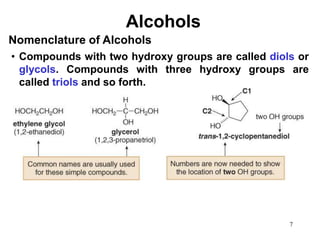
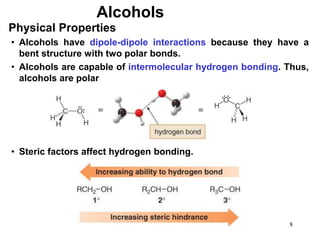
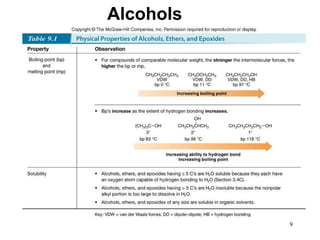
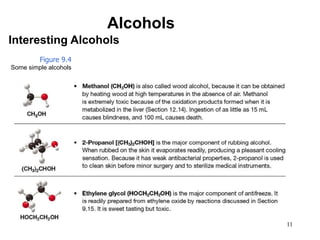
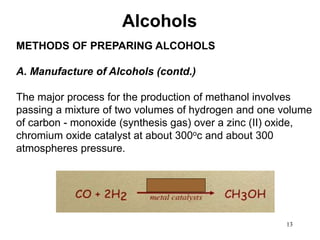
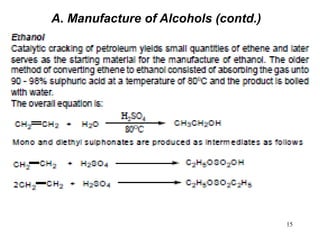
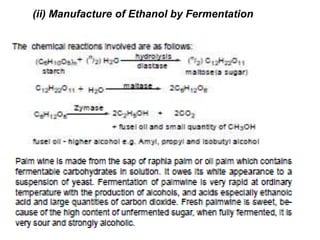
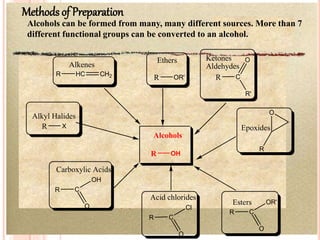
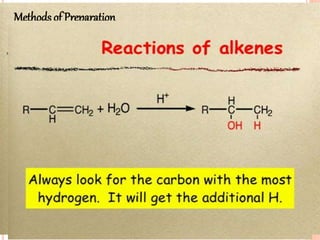
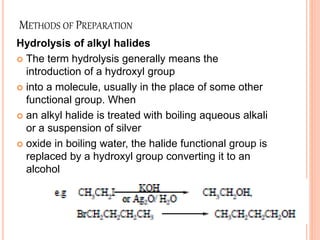
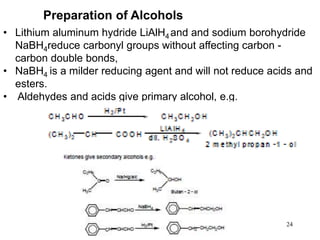
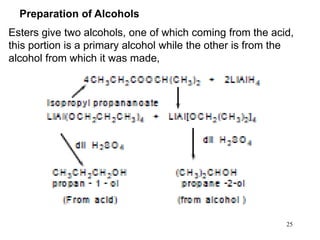
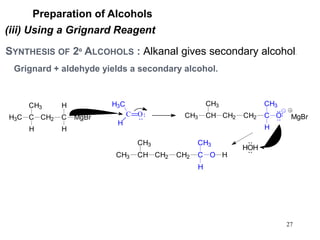
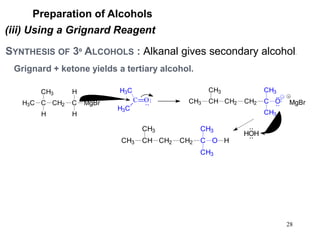
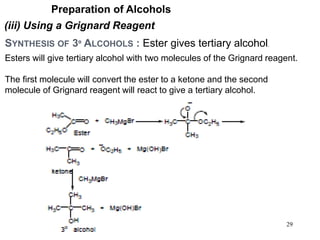
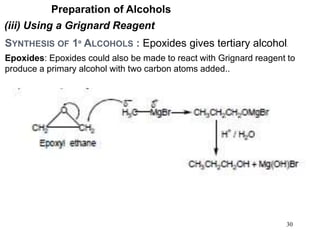
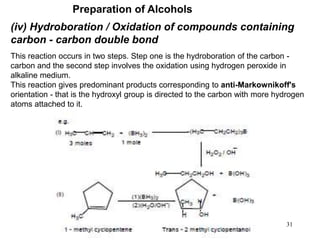
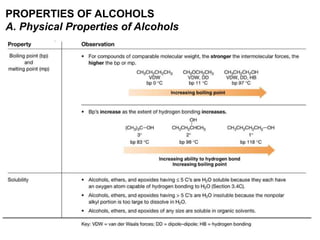
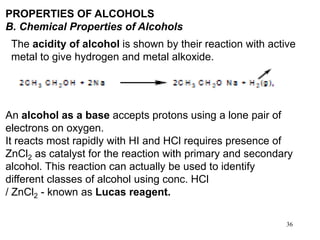
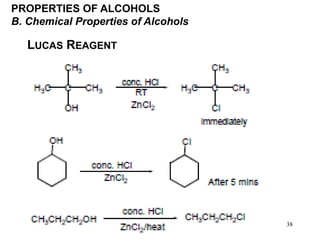
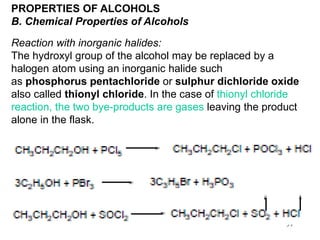
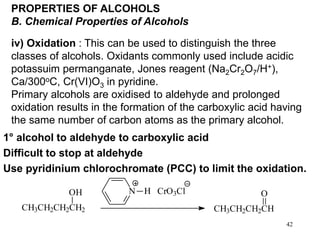
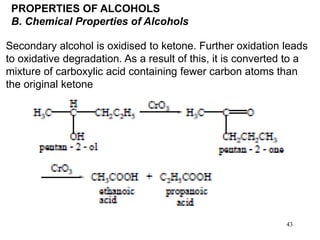
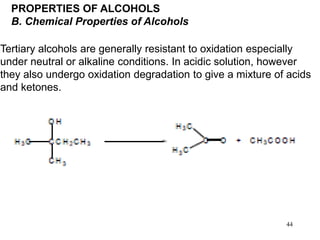
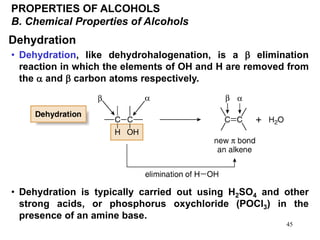
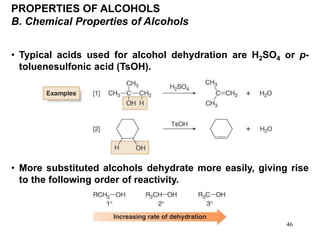
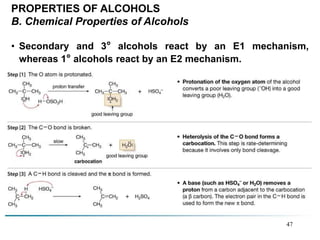
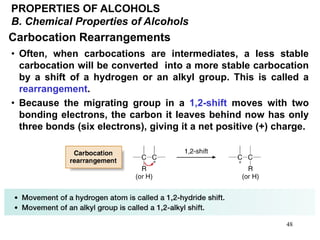
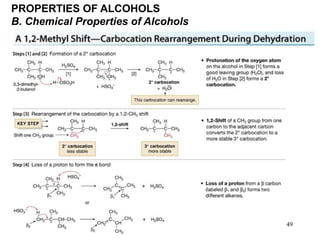
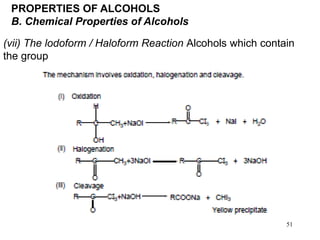
Alcohols can undergo several chemical reactions:
1) They act as both acids and bases due to the hydroxyl group and can form alkoxides or accept protons.
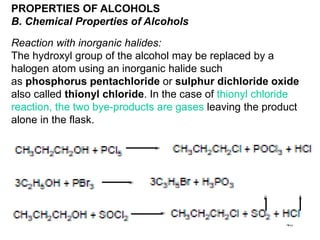
2) The hydroxyl group can be replaced by halides using reagents like phosphorus pentachloride or thionyl chloride.
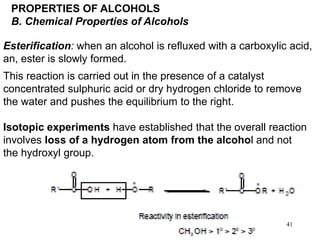
3) Alcohols can undergo esterification when reacted with carboxylic acids to form esters.