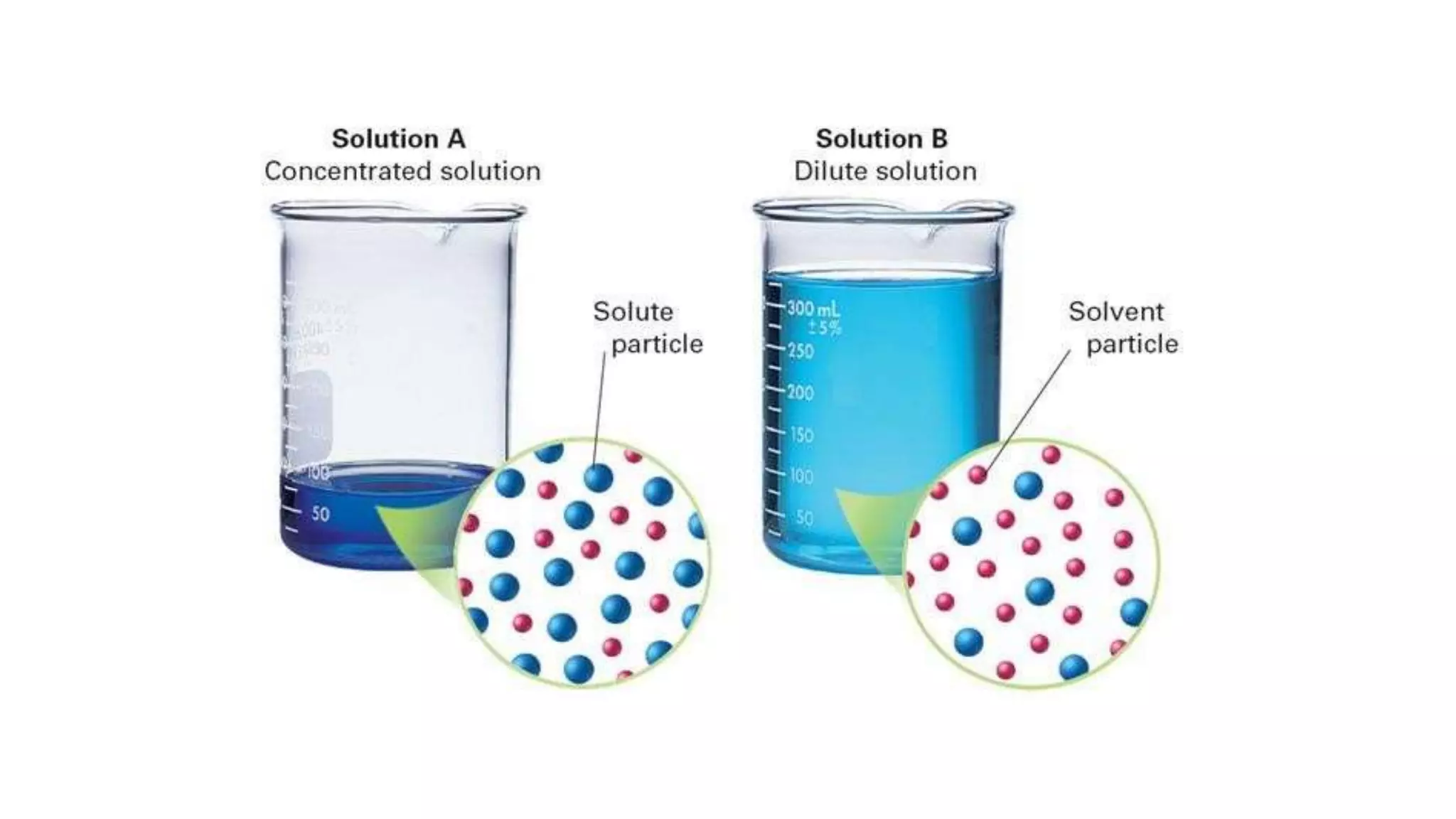
This document discusses different methods of measuring solute concentrations in solutions. It defines key terms like solute, solvent, concentration, and explains common units of concentration like molarity and molality. Molarity is moles of solute per liter of solution, while molality is moles of solute per kilogram of solvent. The document also covers colligative properties, how solutions can be isotonic, hypertonic, or hypotonic, and different ways of expressing drug concentrations including parts ratio, percentage, and weight per volume.