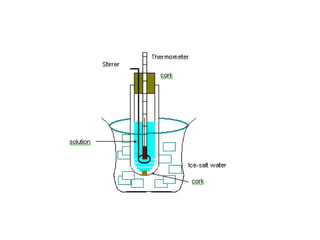
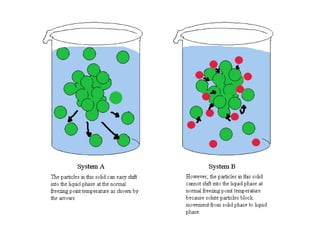
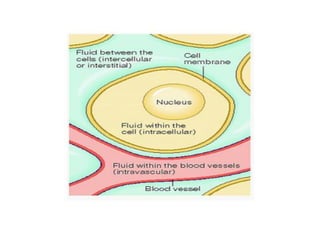
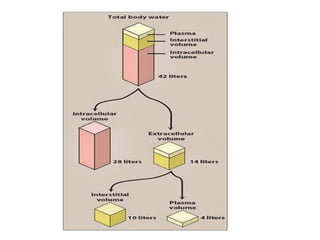
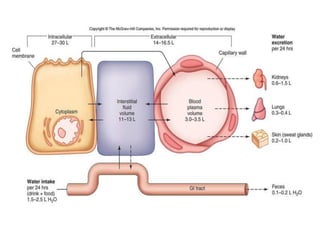
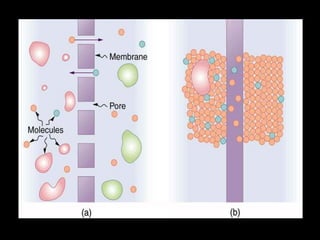
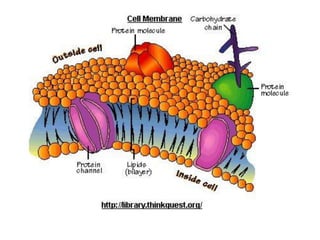
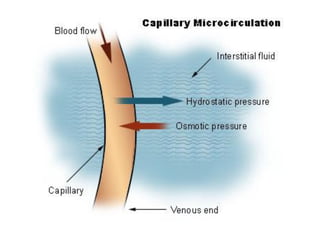
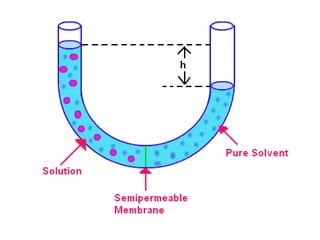
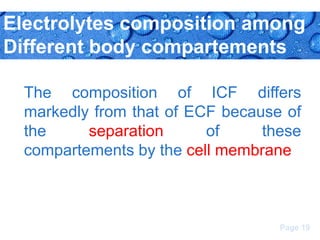
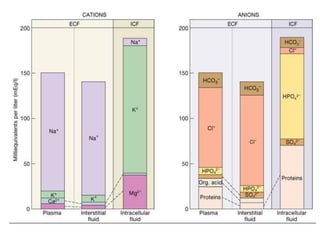
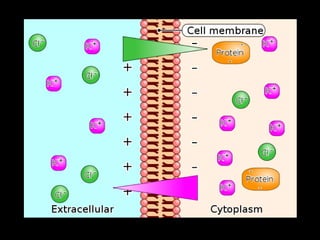
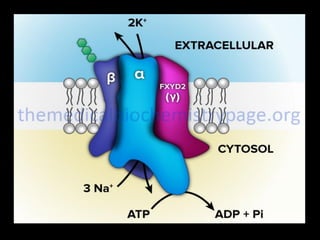
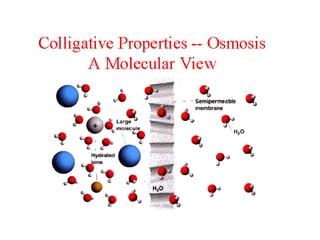
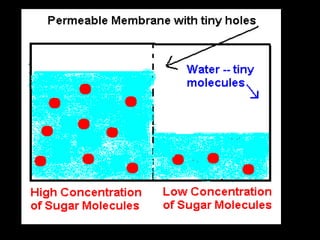
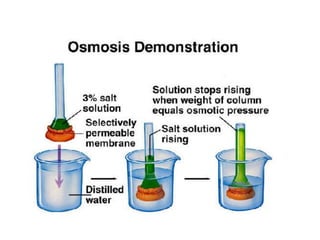
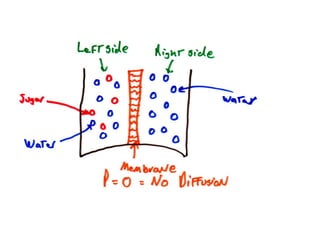
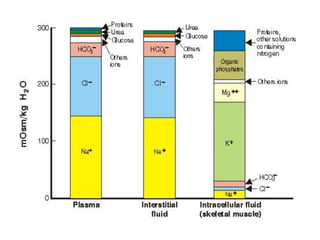
This document discusses body water, osmolality, and related topics. It begins by explaining that total body water decreases from around 90% of body weight in fetuses to around 65% in adult males and 55% in adult females. It then covers Starling forces that govern fluid movement between compartments, osmotic pressure, electrolyte composition differences between intracellular and extracellular fluid, Gibbs-Donnan equilibrium, and active transport mechanisms. The document defines osmolality and osmolarity, explains colligative properties, and discusses clinical significance and methods for measuring plasma and urine osmolality.

























![Calculation of Plasma Osmolality:
mOsm/Kg =
1.86 [Na+(mmol/L)]+Glucose
+urea (mmol/L)+9
(mmol/L)
1.86= Na+ and Cl- (incomplete dissociation)
9: The contribution of other osmotically
active substances in plasma such as K+,
Ca2+, and proteins.
Page 42](https://image.slidesharecdn.com/bodywaterandosmolalitymsc28-10-2013-131028190000-phpapp01/85/Body-water-and-Osmolality-42-320.jpg)