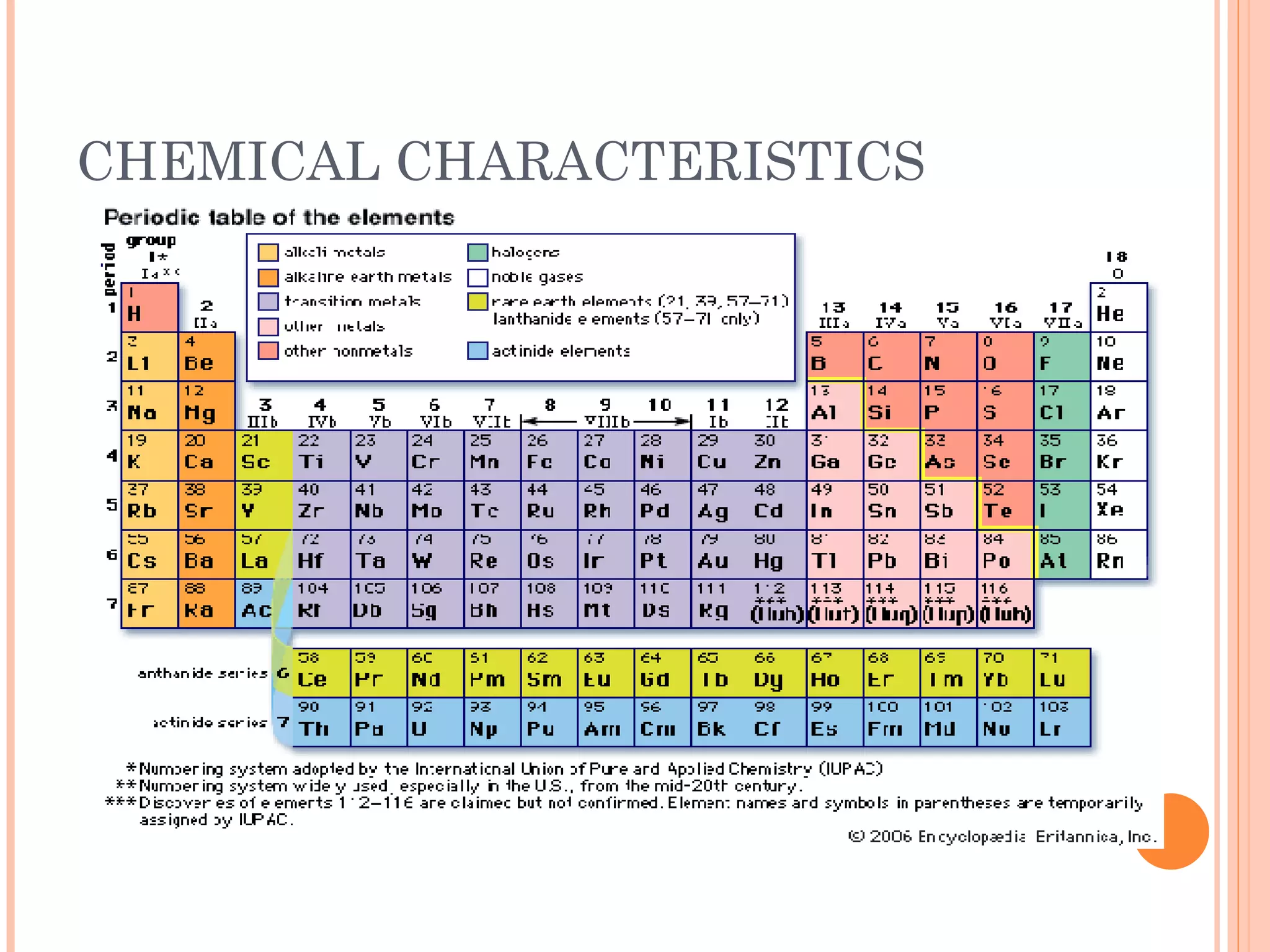
This document discusses chemical characteristics and properties. It defines a chemical change as when two or more substances unite or break apart chemically, resulting in a new substance with different properties than the original. It also defines elements, compounds, atoms, and molecules. Atoms are made up of protons and neutrons in the nucleus and electrons orbiting the nucleus. The periodic table arranges elements by their atomic number and symbol.