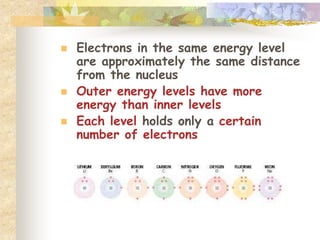
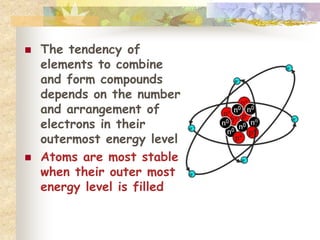
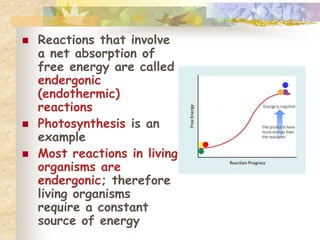
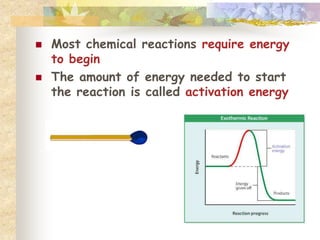
This document provides an overview of chemistry concepts including:
- Matter is anything that occupies space and has mass. Atoms are the smallest particle of an element and consist of protons, neutrons, and electrons.
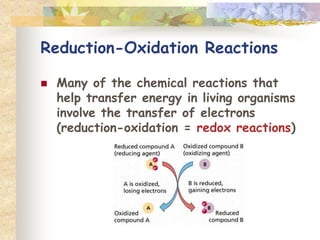
- Elements combine in fixed proportions to form compounds. Chemical reactions involve breaking and forming bonds to create new substances.
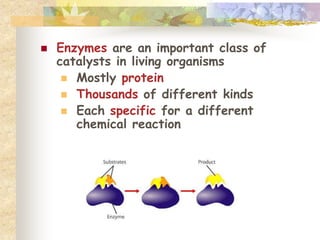
- Living things require energy, obtained through exergonic reactions like cellular respiration. Catalysts like enzymes lower reaction activation energies.
- Solutions are uniform mixtures where particles of solute are distributed within a solvent. Aqueous solutions in living things are important for biochemical reactions. Acids and bases are defined by hydronium and hydroxide ion concentrations measured on the pH scale.