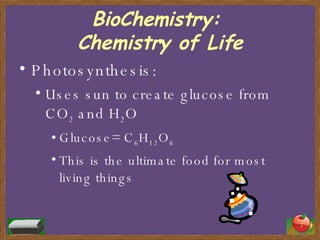
The document provides an overview of biochemistry and the key components of cells. It discusses the four main compounds that make up cells - carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates like glucose are used for energy, lipids include fats and oils, proteins perform important functions and are made of amino acids, and nucleic acids DNA and RNA control cell activities and inheritance. The document also describes important cell structures like mitochondria and the nucleus, as well as metabolic processes like cellular respiration.