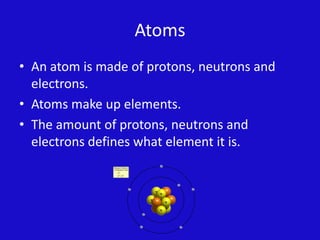
Elements are made up of atoms that contain protons, neutrons and electrons. Atoms combine to form compounds made of two or more elements that can be separated through chemical reactions. Chemical reactions involve elements interacting to form new compounds, which can sometimes produce byproducts like gases. A chemical change alters the substances chemically while a physical change only modifies the shape or form. Reaction rates can be affected by factors like temperature, with heat speeding them up and cold slowing them down.