1. The document provides definitions and explanations of key concepts in chemistry and biochemistry, including atoms, atomic number, mass number, isotopes, the periodic table, valence, molecules, elements, compounds, molecular mass, moles, molarity, normality, and molality.
2. It explains that chemistry is the study of matter and its interactions, while biochemistry focuses on chemical processes within living organisms and their molecules like proteins, nucleic acids, carbohydrates and lipids.
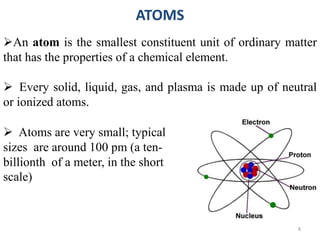
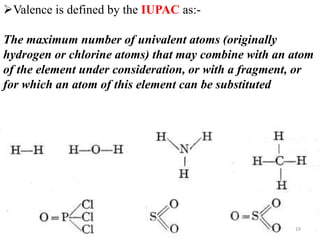
3. Key points covered include that atoms are the smallest units of matter, the periodic table arranges elements by atomic number, valence indicates an element's combining power, and molecules, elements, and compounds are distinguished based on