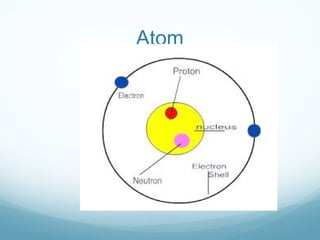
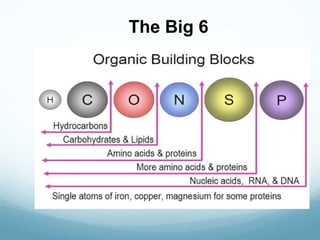
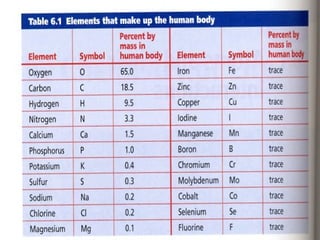
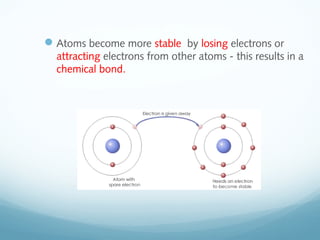
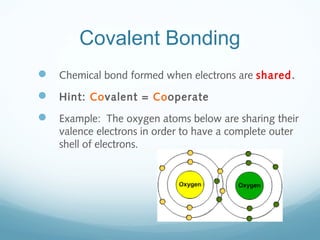
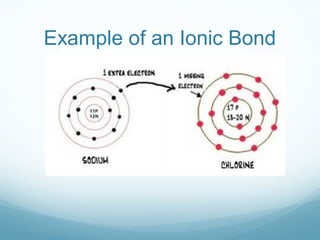
The document discusses basic chemistry concepts including the composition of matter at the atomic level, elements, compounds, and bonding. It explains that atoms are made up of protons, neutrons, and electrons. Elements are pure substances made of one type of atom, with 92 naturally occurring elements and 26 found in living things. The four main elements that make up nearly all of an organism's weight are hydrogen, oxygen, nitrogen, and carbon. Atoms can bond through ionic bonds by gaining or losing electrons, or covalent bonds by sharing electrons, in order to achieve stable full energy levels.