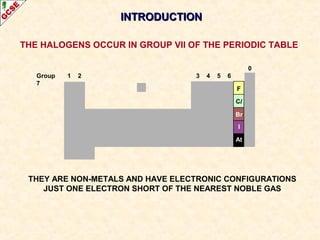
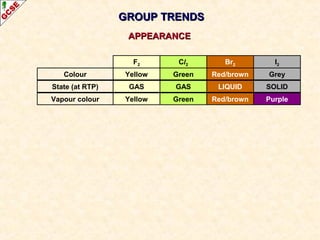
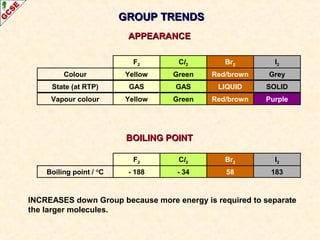
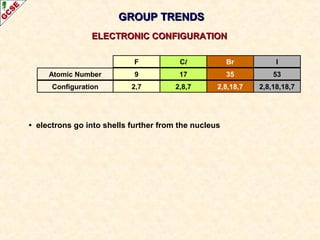
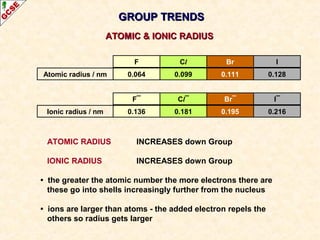
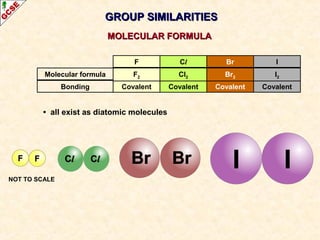
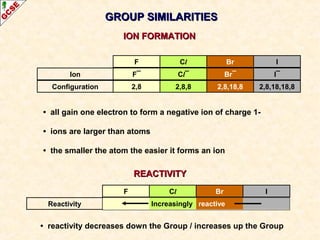
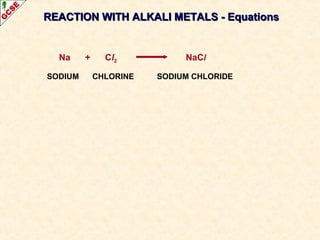
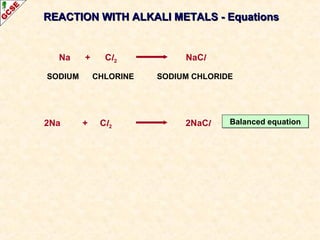
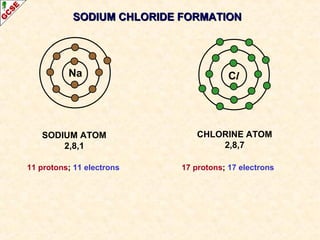
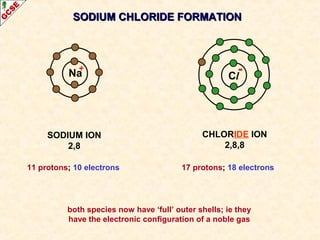
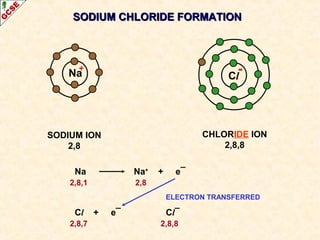
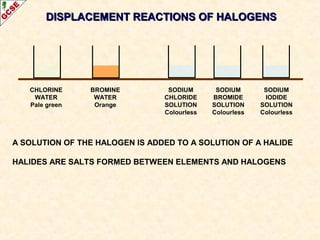
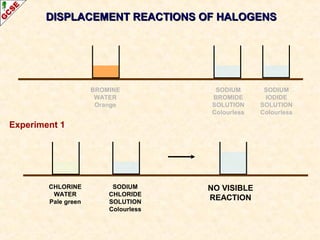
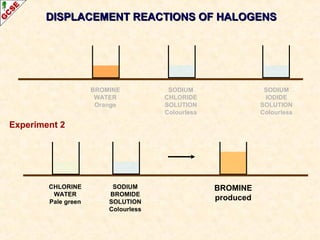
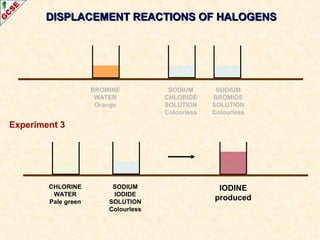
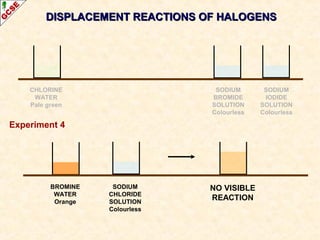
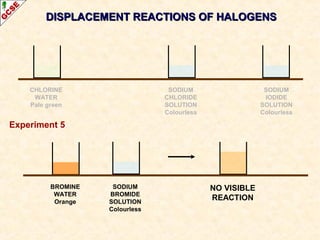
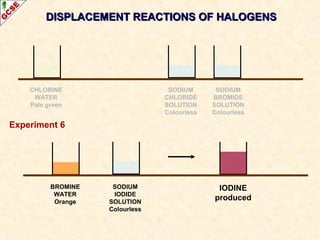
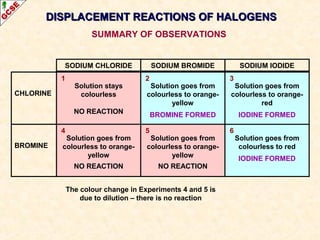
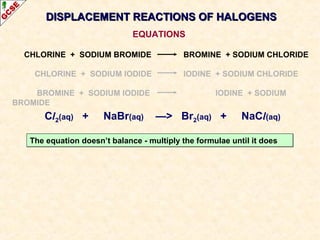
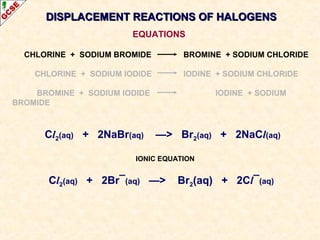
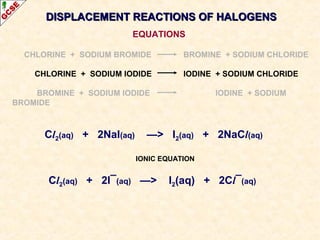
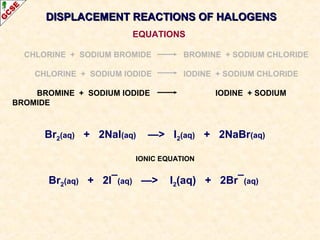
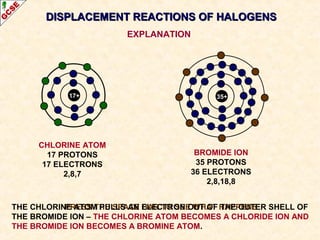
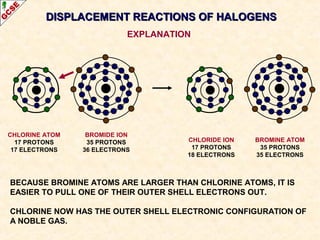
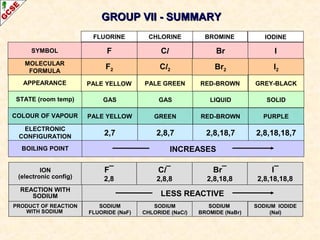
The document is a guide about the halogens for GCSE chemistry students. It discusses the key properties and trends within group VII of the periodic table. The guide covers topics such as physical appearances, boiling points, electronic configurations, atomic sizes, and reactivity trends. It also describes reactions of halogens with metals and displacement reactions between halides.