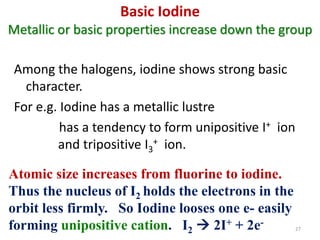
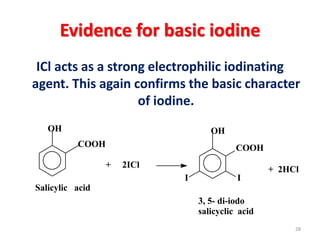
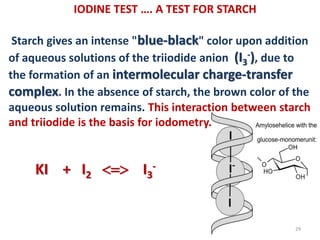
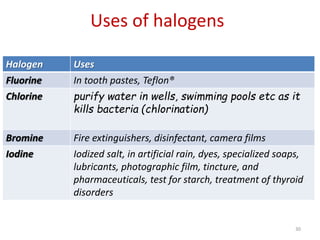
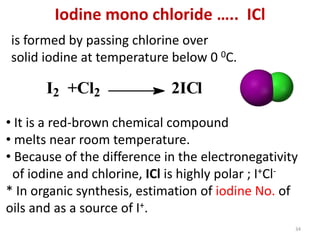
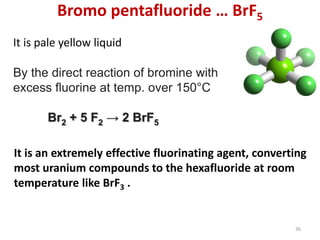
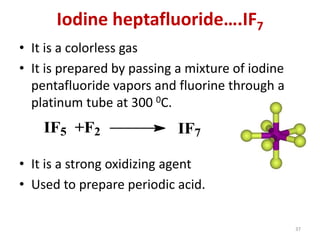
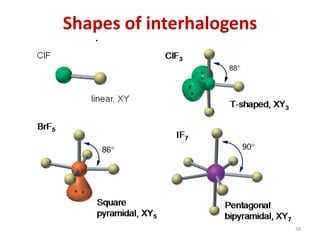
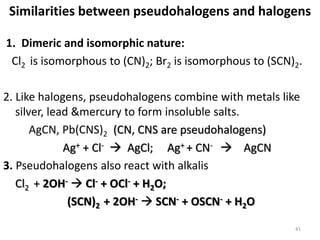
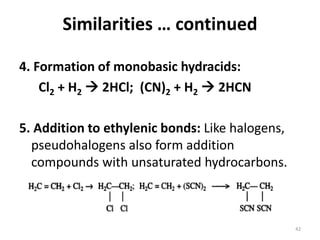
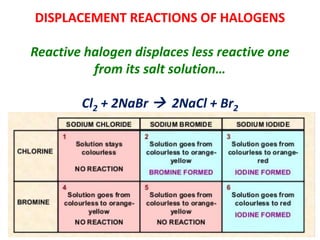
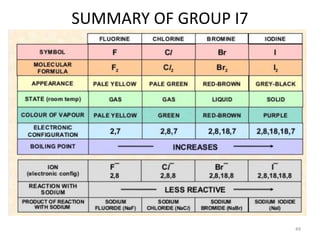
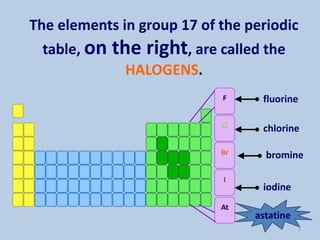
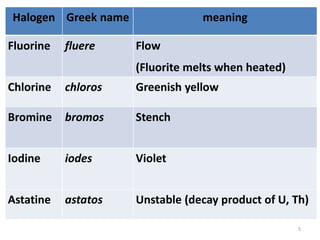
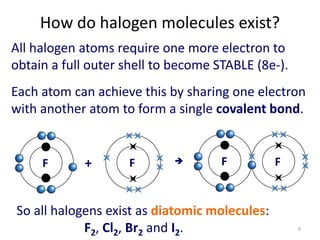
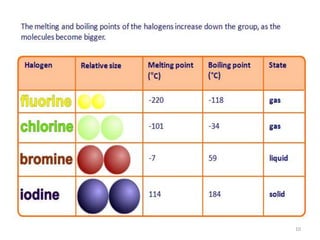
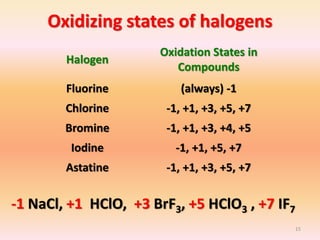
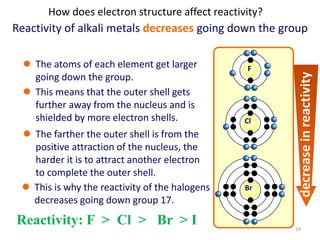
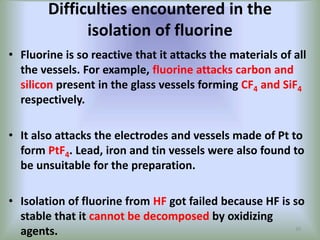
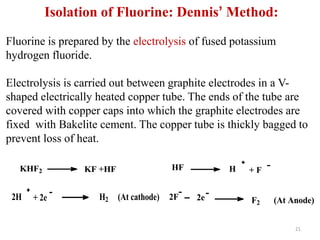
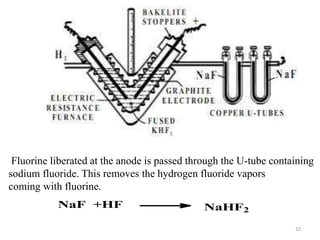
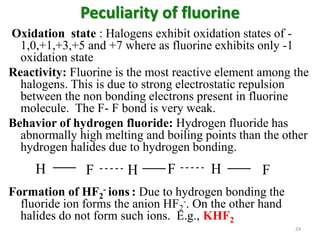
The document discusses the halogens, which are the elements in group 17 of the periodic table (fluorine, chlorine, bromine, iodine, astatine). It provides details about their general properties, including their electron configuration, existence as diatomic molecules, colors, reactivity, and ability to gain electrons to achieve stable electronic structures. It also describes their physical and chemical properties such as ionization energy, electronegativity, electron affinity, and oxidizing power (which decreases down the group). Peculiar properties of fluorine are highlighted. Uses of halogens and facts about their presence in humans are also mentioned. Interhalogen compounds and pseudohalides/pseudohalogens




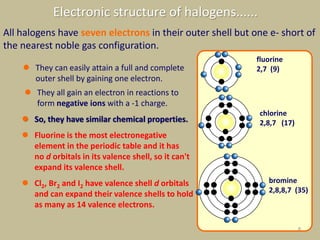
![Electron configuration of halogens
Fluorine 1s2 2s2 2p5
Chlorine [Ne]3s2 3p5
Bromine [Ar]3d10 4s2 4p5
Iodine [Kr]4d10 5s2 5p5
Astatine [Xe]4f14 5d10 6s2 6p5
7](https://image.slidesharecdn.com/fascinatingaspectsofhalogens-171219054601/85/Aspects-of-halogens-7-320.jpg)








![Peculiarity of fluorine ….. continued
• Maximum covalency: As fluorine lacks d orbitals it
cannot have covalency more than one. But the other
halogens can have covalency up to 7. (eg. IF7)
• Formation of SF6: Due to small size and high
electronegativity, fluorine brings about the + 6 state in
sulphur forming SF6. Other halogens do not form such
hexahalides
• Complex formation: Flouride ion a greater tendency to
form [FeF6]3-, [AlF6]3-
• Formation of polyhalides: Due to the non-availability
of d-orbitals fluorine does not form any polyhalides. But
other halogens form polyhalide ions. E.g. [Br2]+, [I2]+ ,
[Cl3]+, [Br3]+, [I3]+ [I3]−
, [Br4]2−, [I4]2−etc 25](https://image.slidesharecdn.com/fascinatingaspectsofhalogens-171219054601/85/Aspects-of-halogens-25-320.jpg)