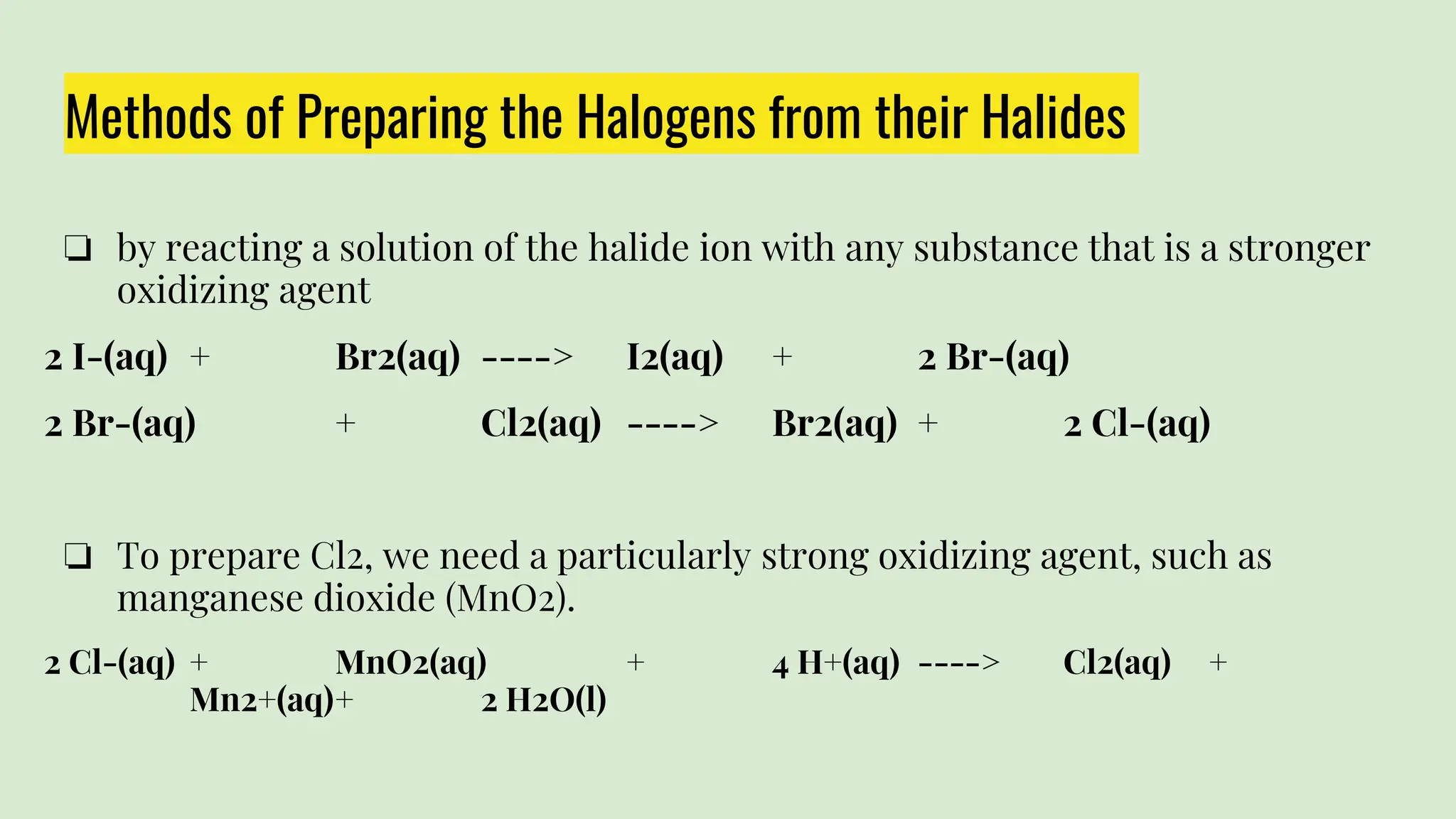
The document summarizes the properties of the halogens. It describes that the six halogens - fluorine, chlorine, bromine, iodine and astatine - all form diatomic molecules and negatively charged ions. They are salt formers and are not found in their elemental form in nature. The document then discusses the specific properties of each halogen in their elemental form and how they are prepared from their halides. It also notes that properties regularly increase or decrease going down the group.