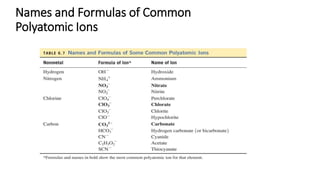
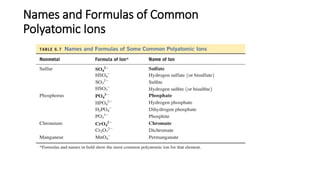
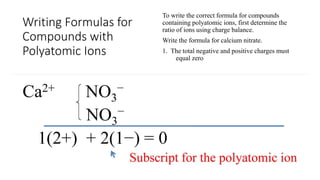
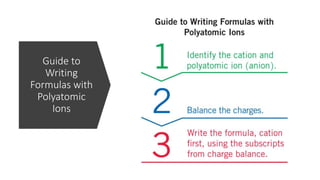
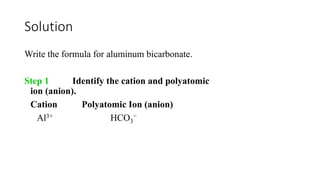
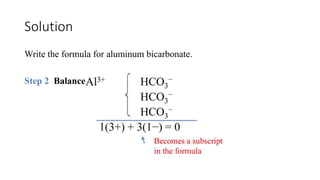
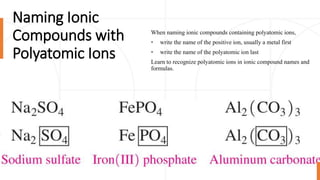
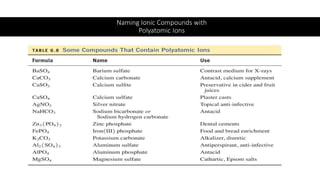
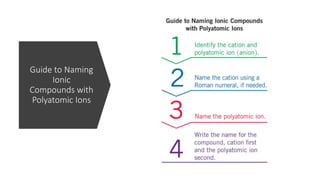
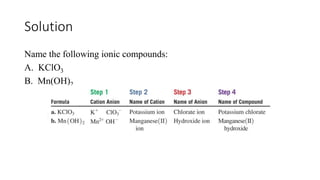
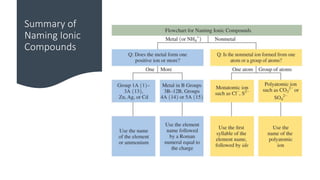
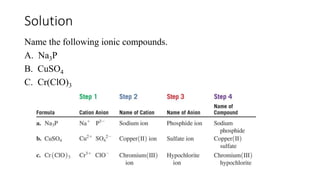
This document discusses polyatomic ions and writing formulas for ionic compounds containing polyatomic ions. It defines polyatomic ions as groups of atoms with an overall ionic charge. Common polyatomic ions such as ammonium, hydroxide, nitrate, and phosphate are presented. The document provides examples of writing formulas for ionic compounds using polyatomic ions by balancing charges between cations and anions. It also discusses naming ionic compounds containing polyatomic ions by writing the name of the metal cation first followed by the polyatomic ion.