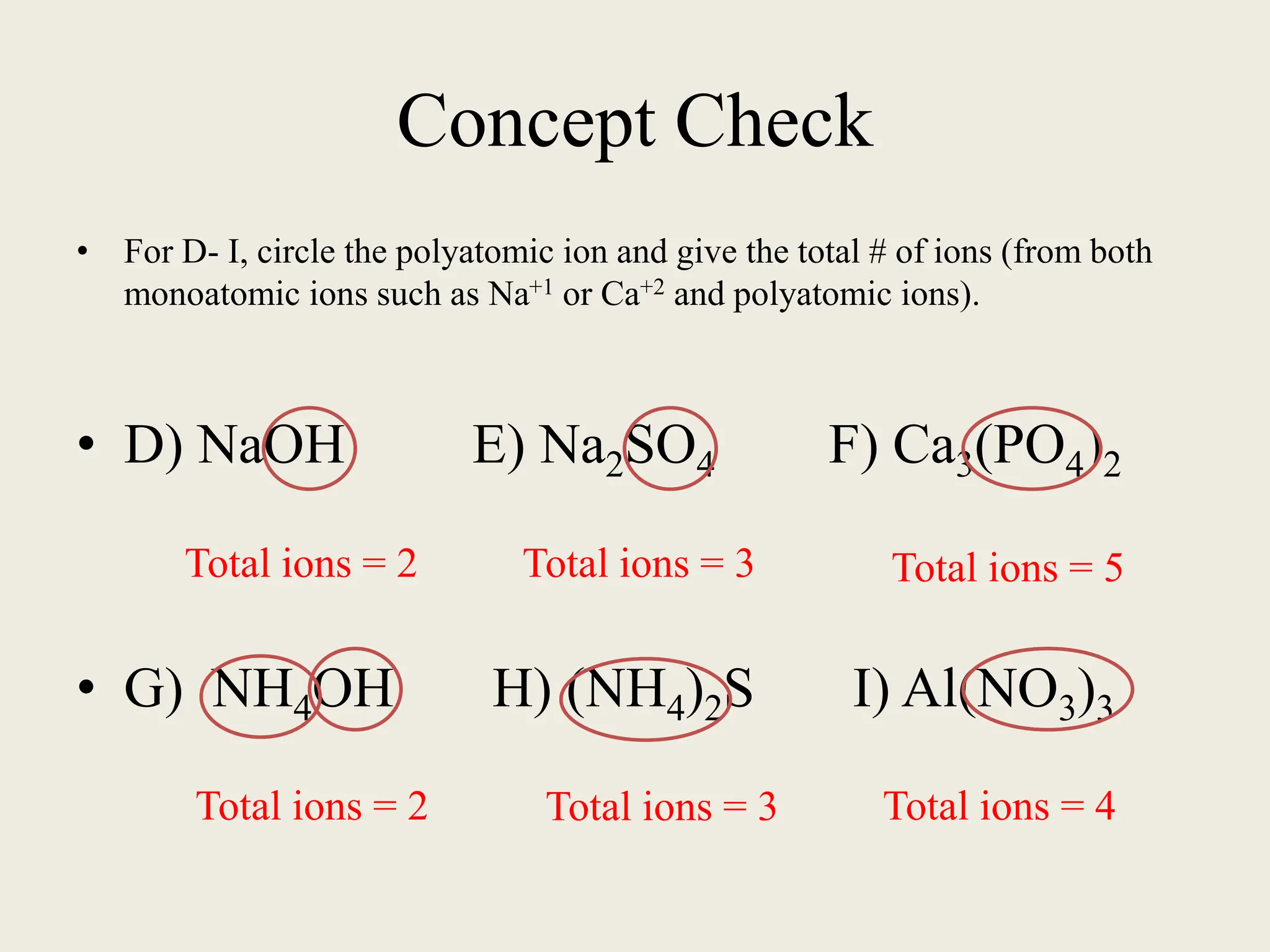
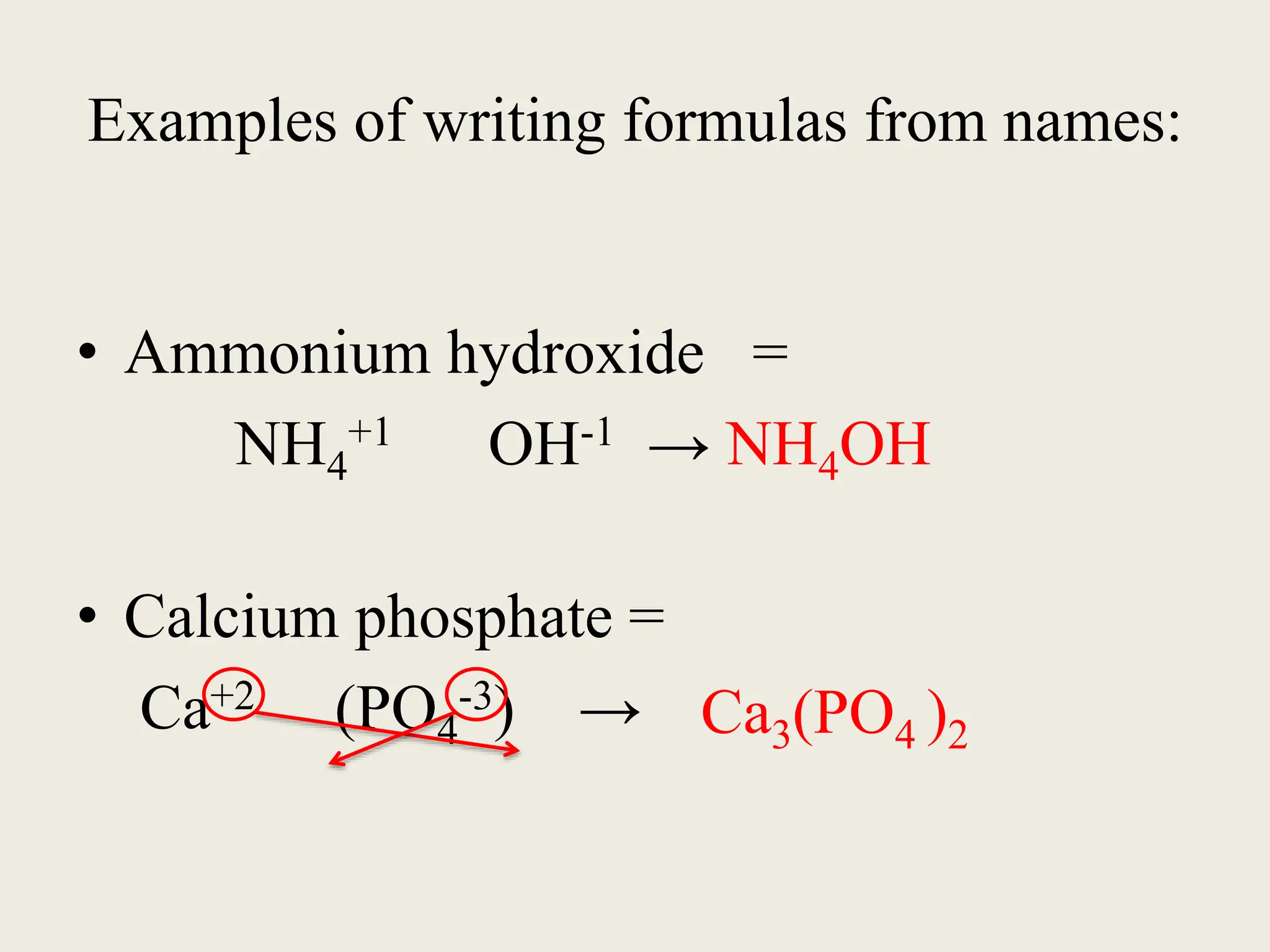
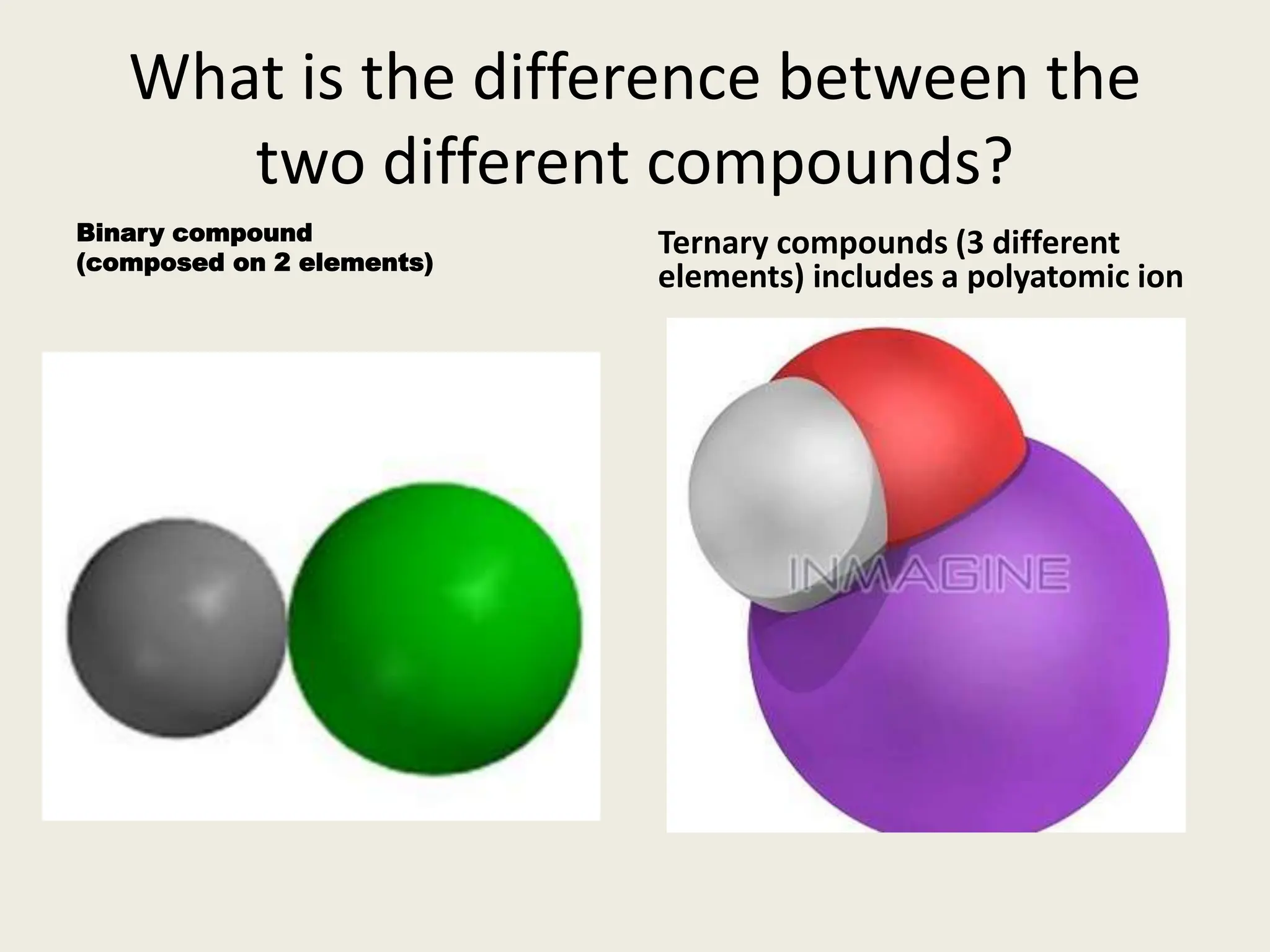
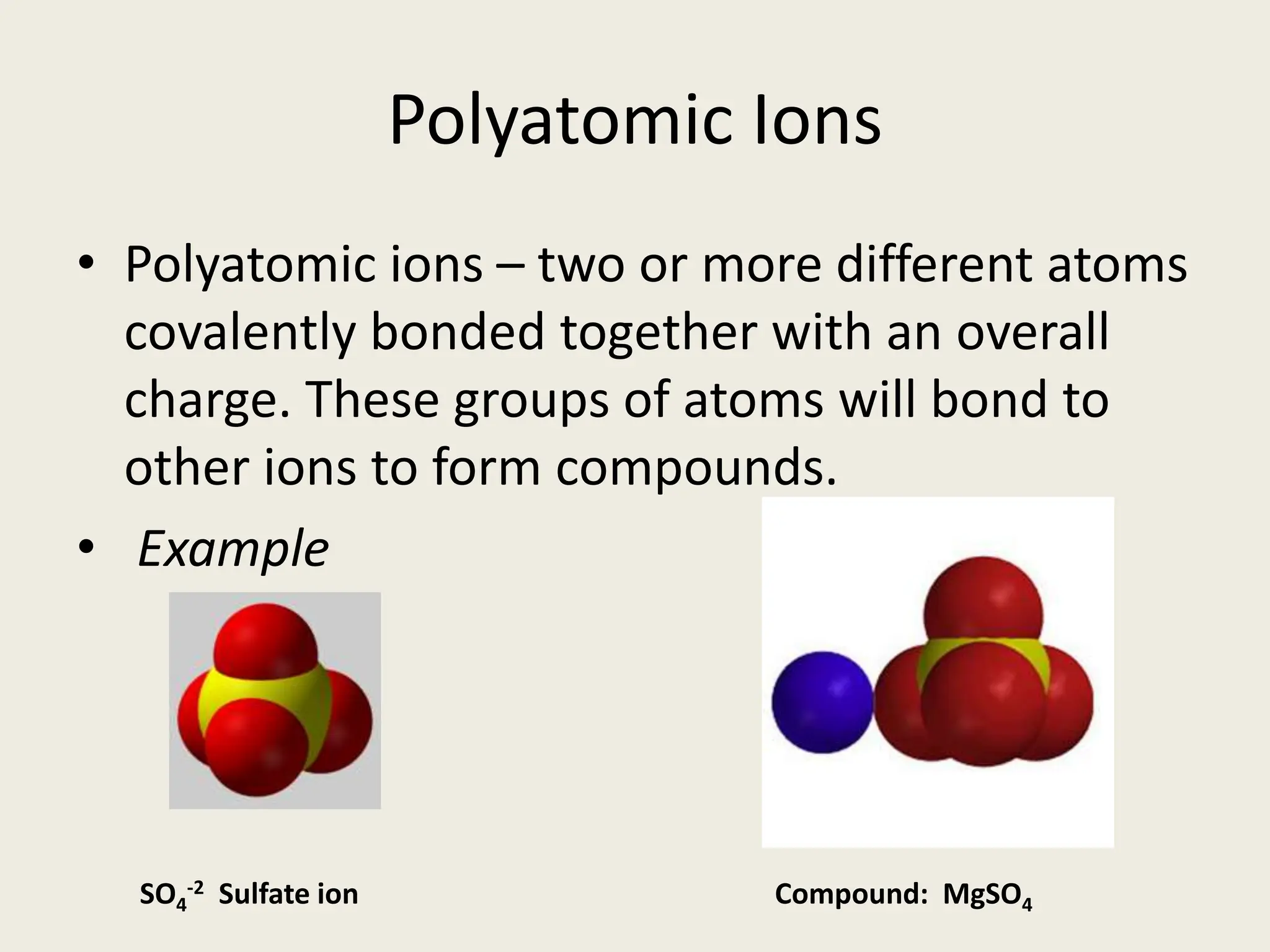
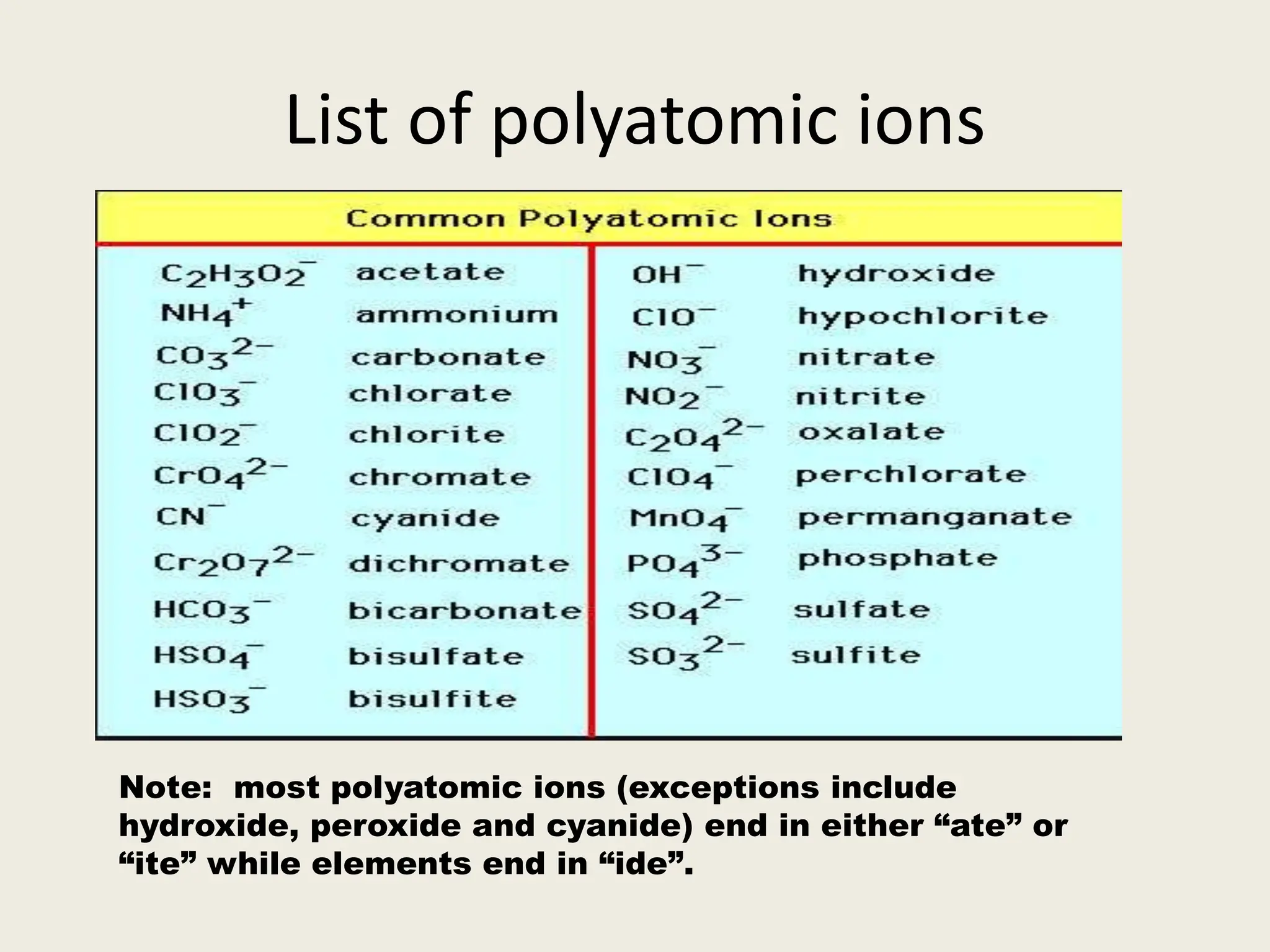
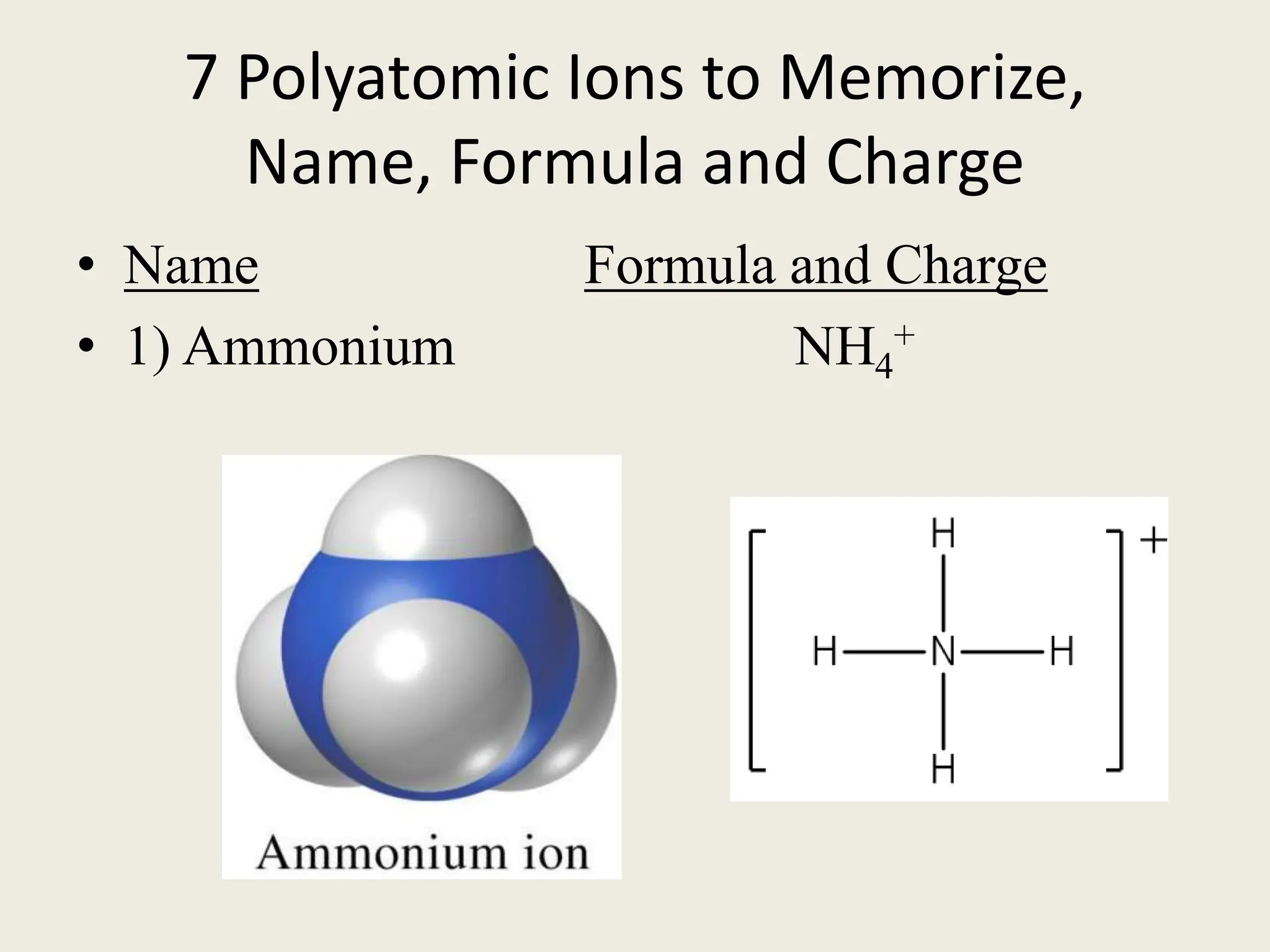
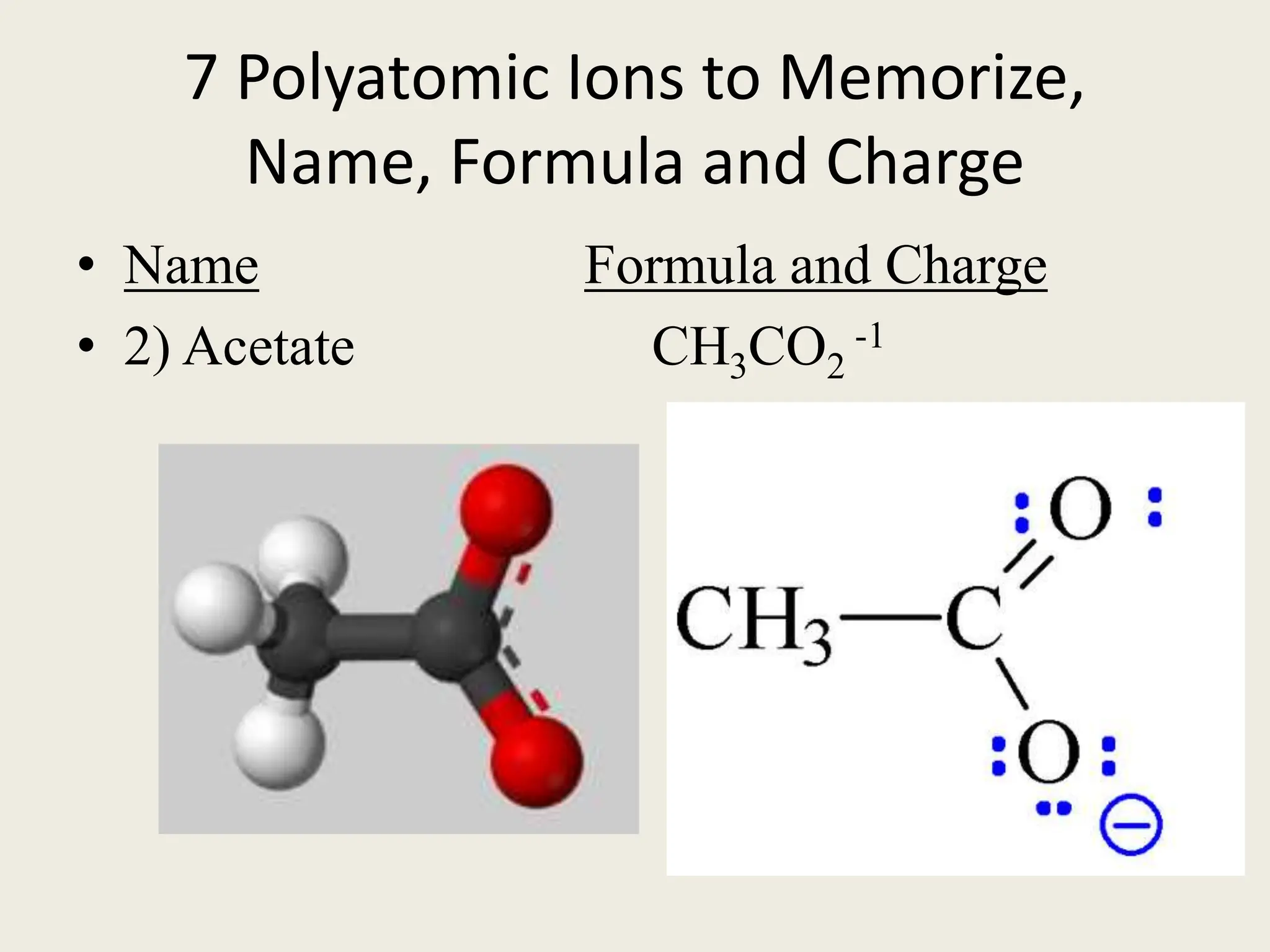
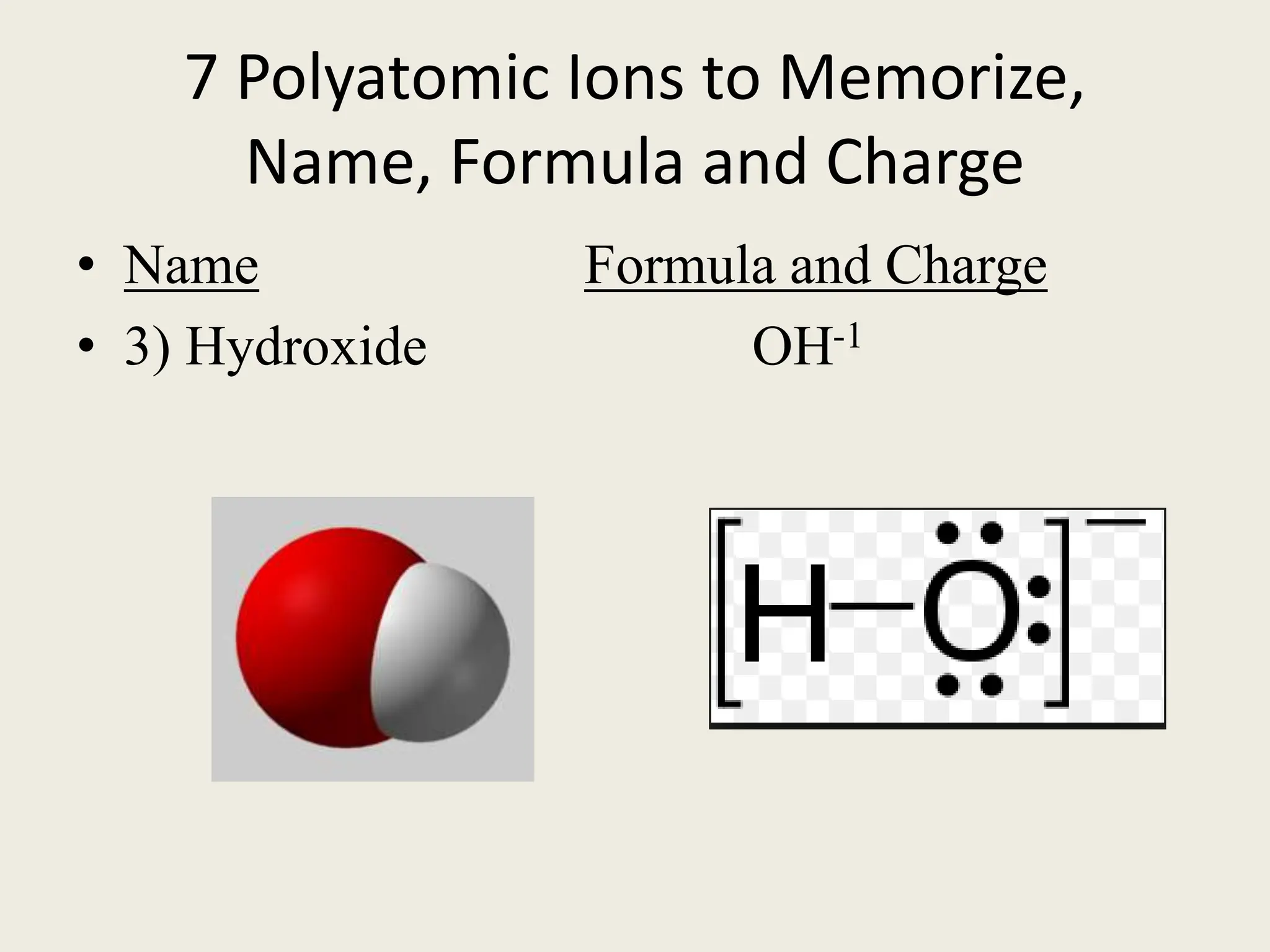
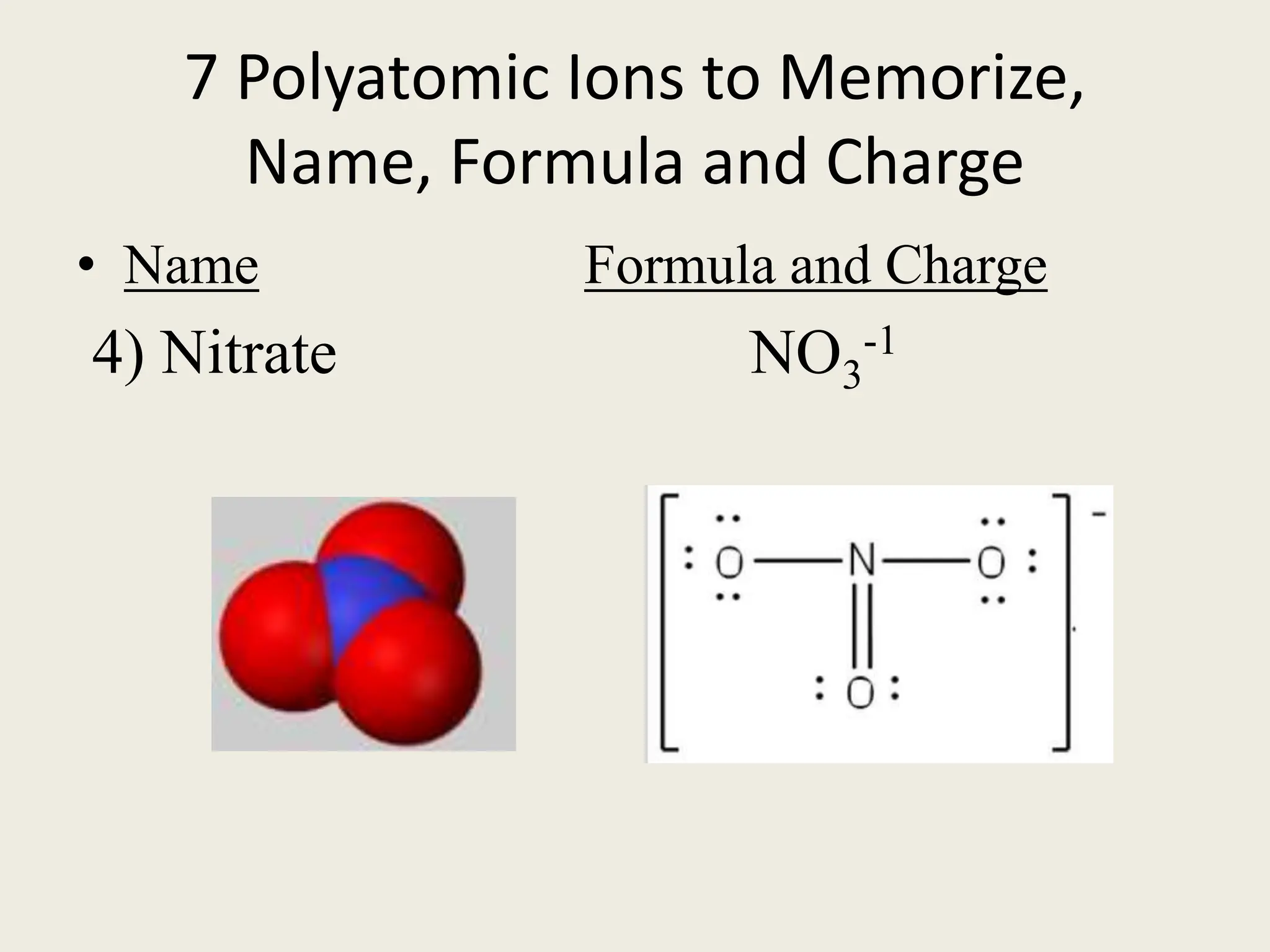
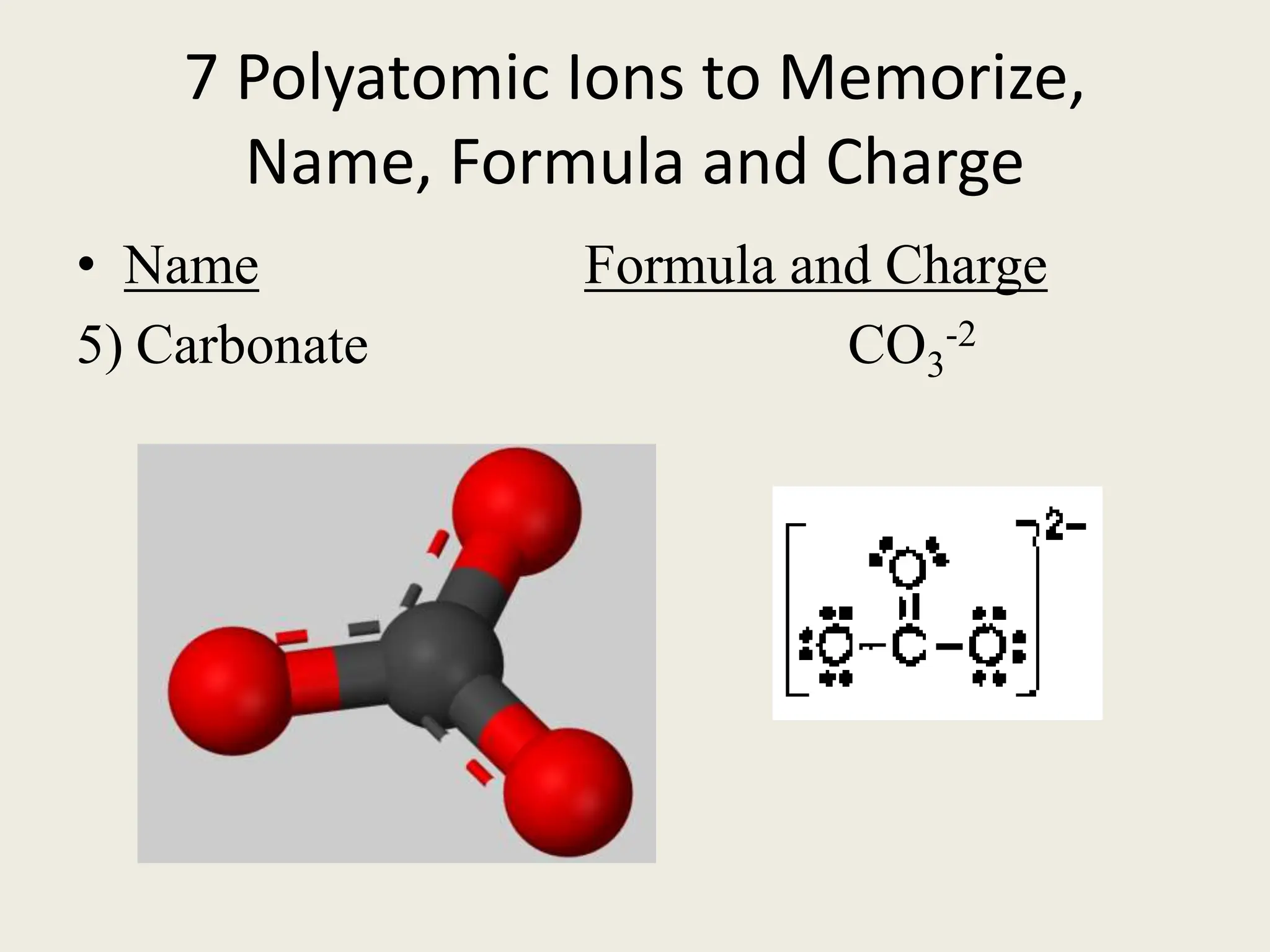
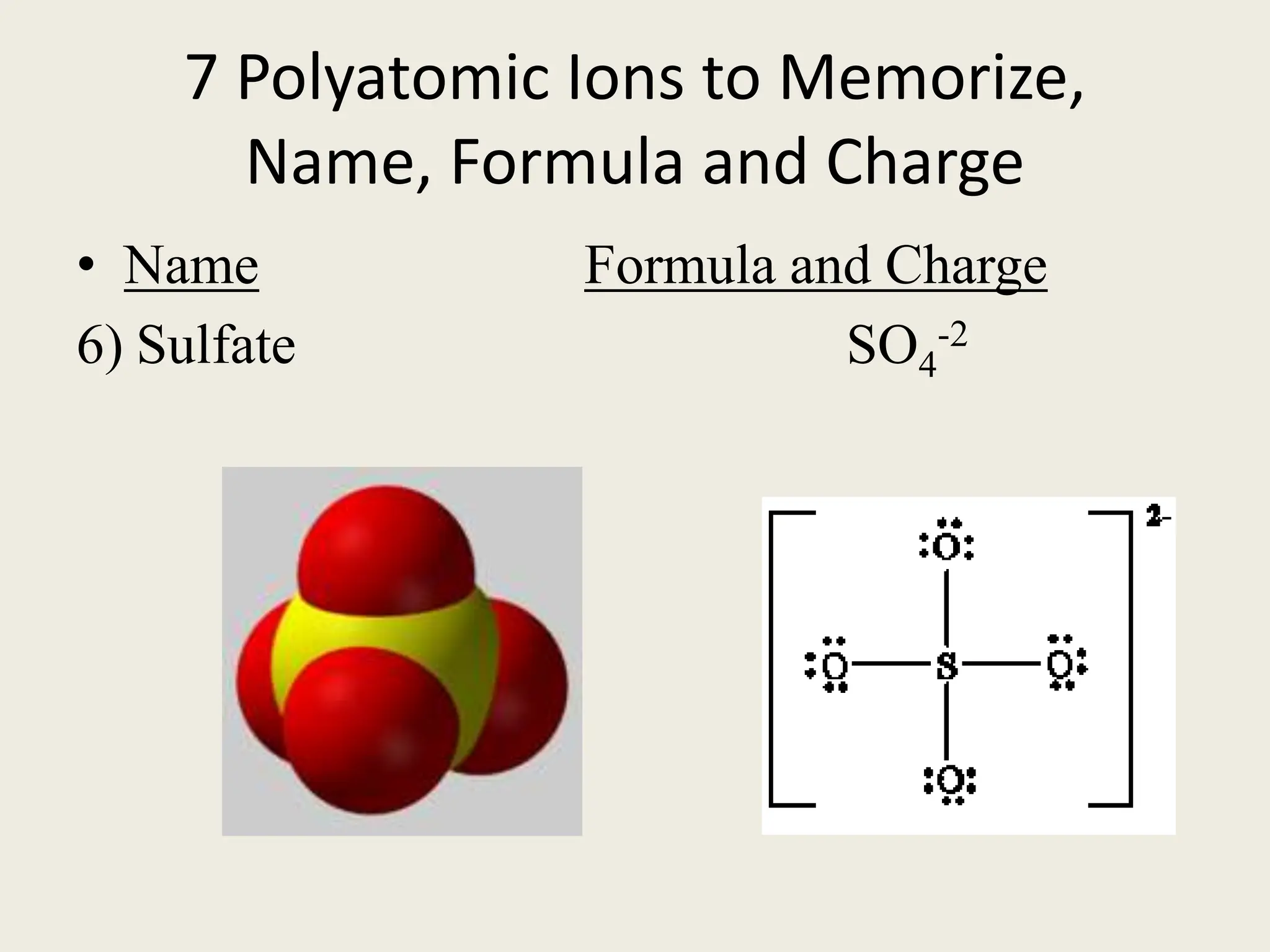
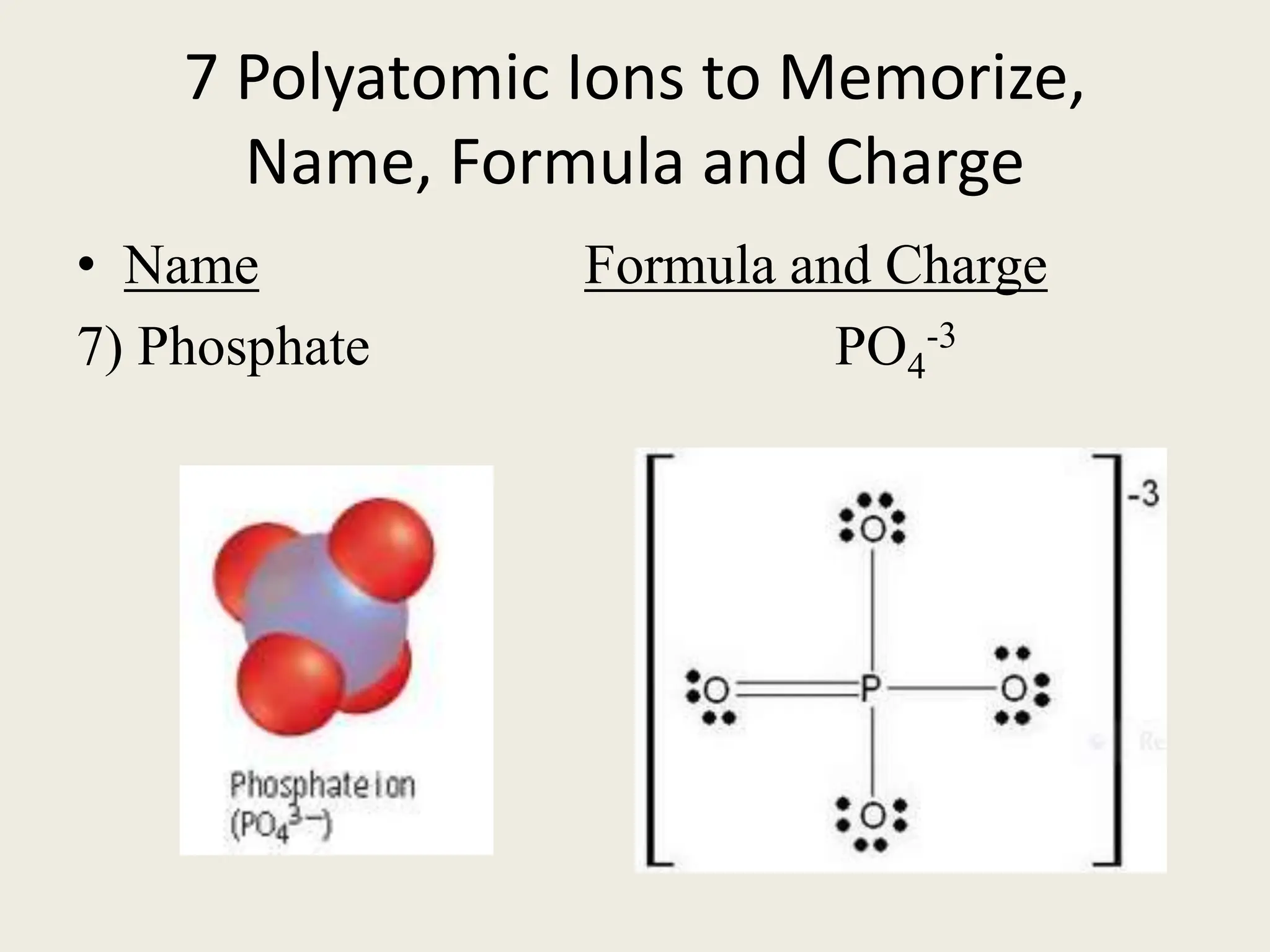
The document explains the differences between binary and ternary compounds, detailing the role of polyatomic ions and providing examples. It includes a list of seven polyatomic ions with their names, formulas, and charges, as well as practical exercises on identifying ions and naming compounds. The document also outlines rules for naming ternary salts and writing chemical formulas, emphasizing the importance of charge balance.
![Concept Check: How many IONS are present
in each of the following?
• (Hints for a-c each ion is enclosed in [ ];
• A) [K]+[OH]-
• B) [Na]+[CO3
-2 ] [Na]+
C) [Na]+ [PO4]-3 [Na]+
[Na]+
Ans: 2
Ans: 3
Ans: 4](https://image.slidesharecdn.com/polyatomic-ions-powerpoint-240419104704-fac76b5f/75/Polyatomic-Ions-What-is-the-difference-between-the-two-different-compounds-pptx-12-2048.jpg)