This document provides information about naming and writing formulas for different types of chemical compounds including:
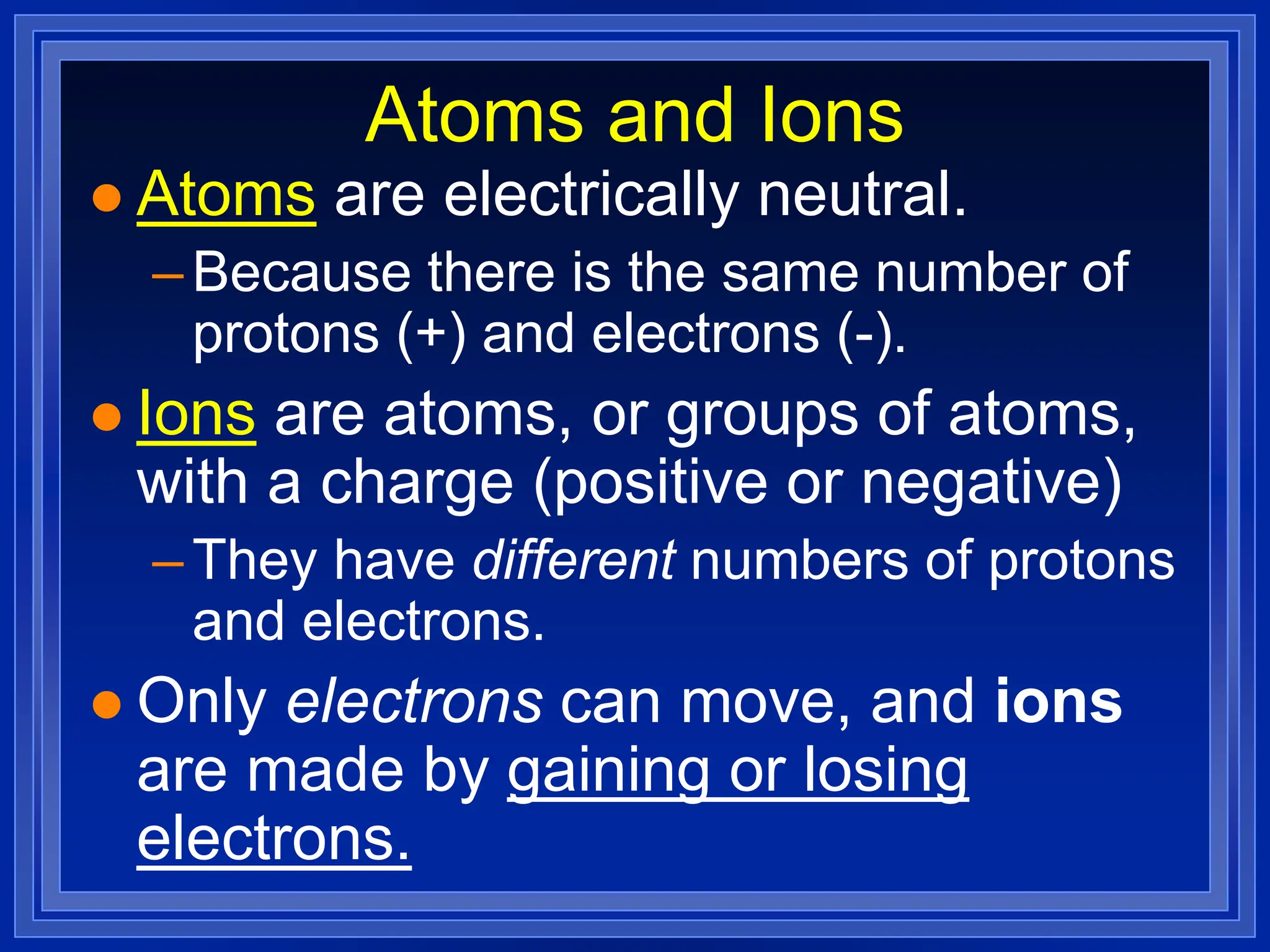
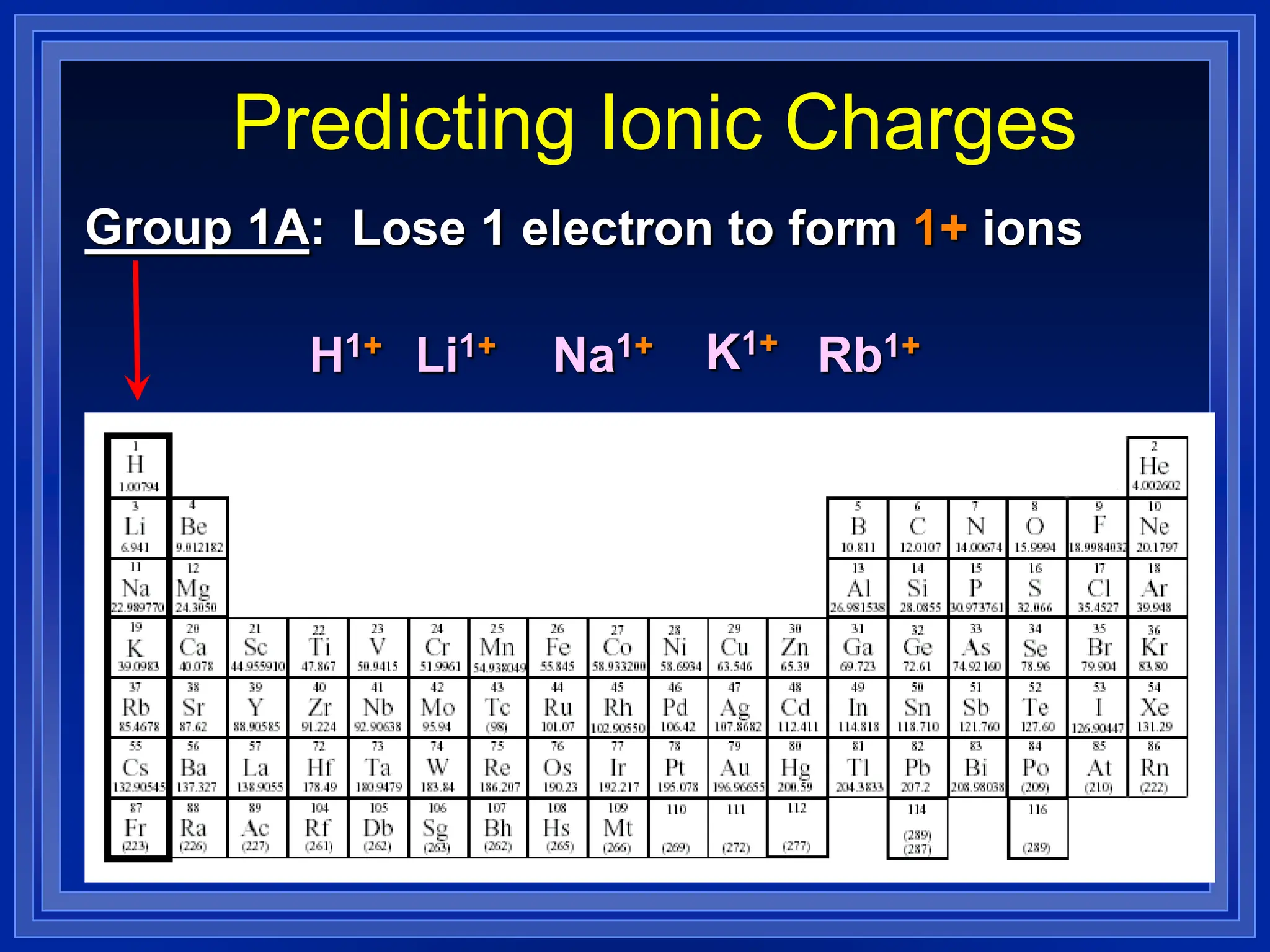
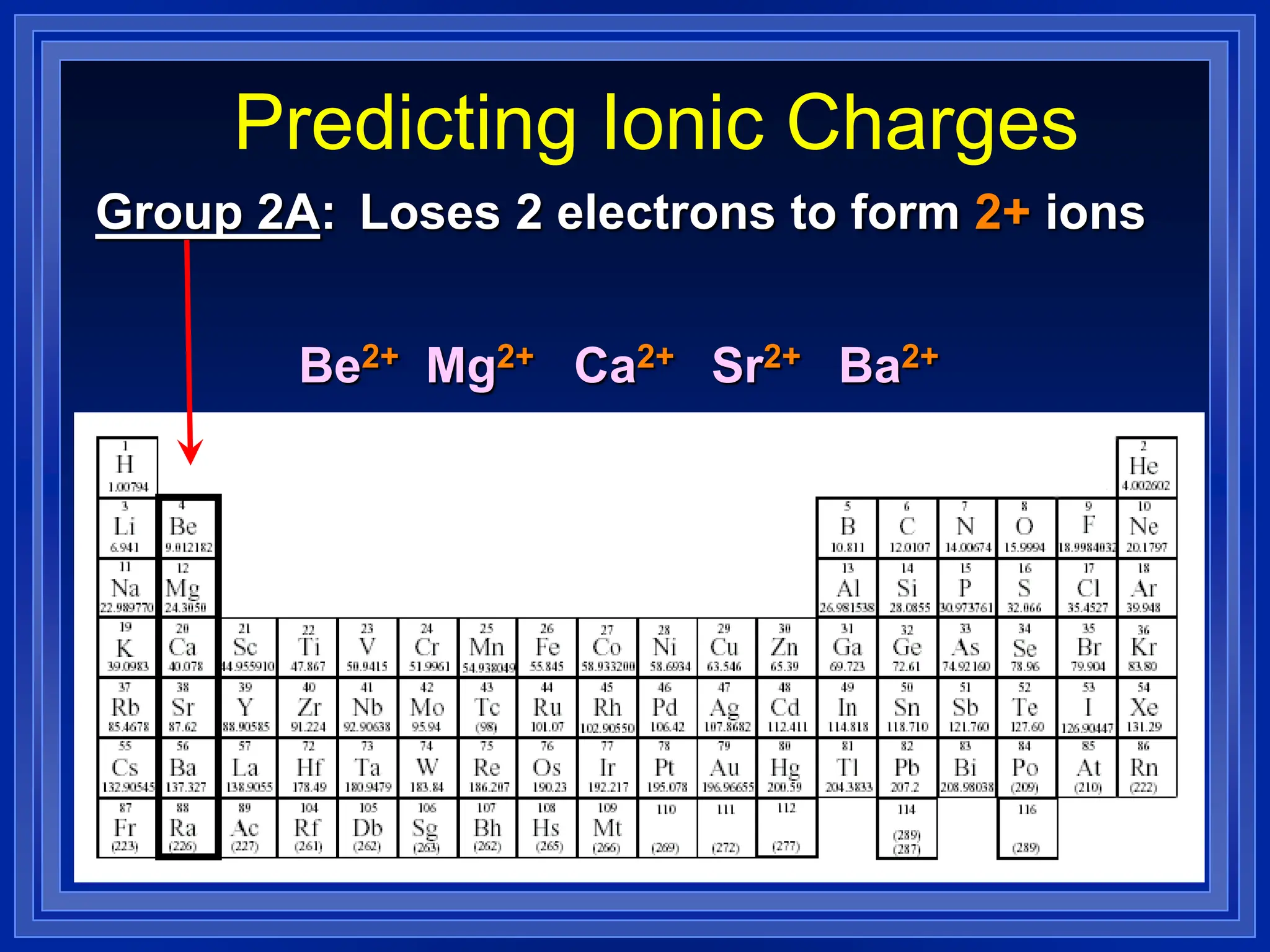
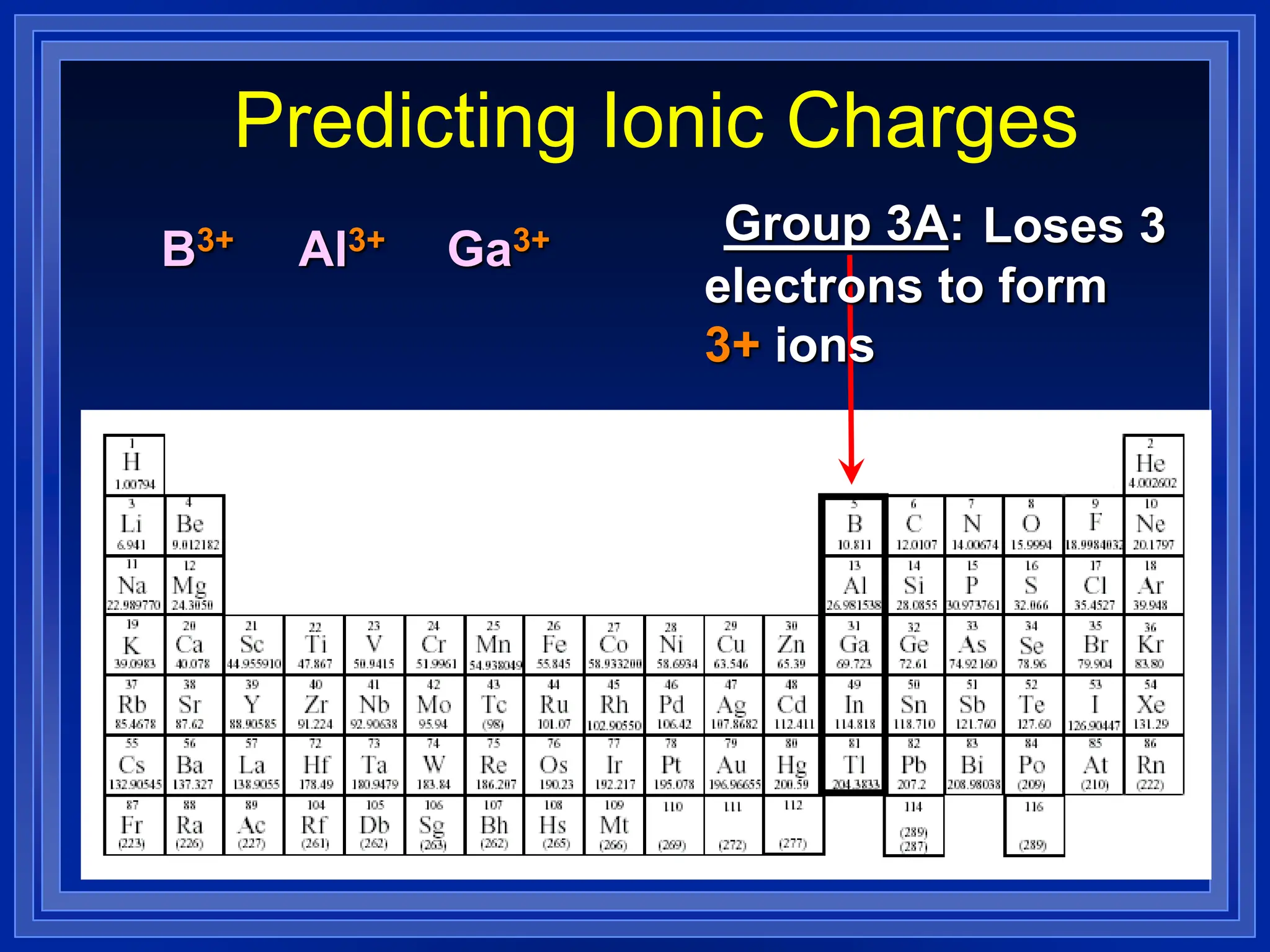
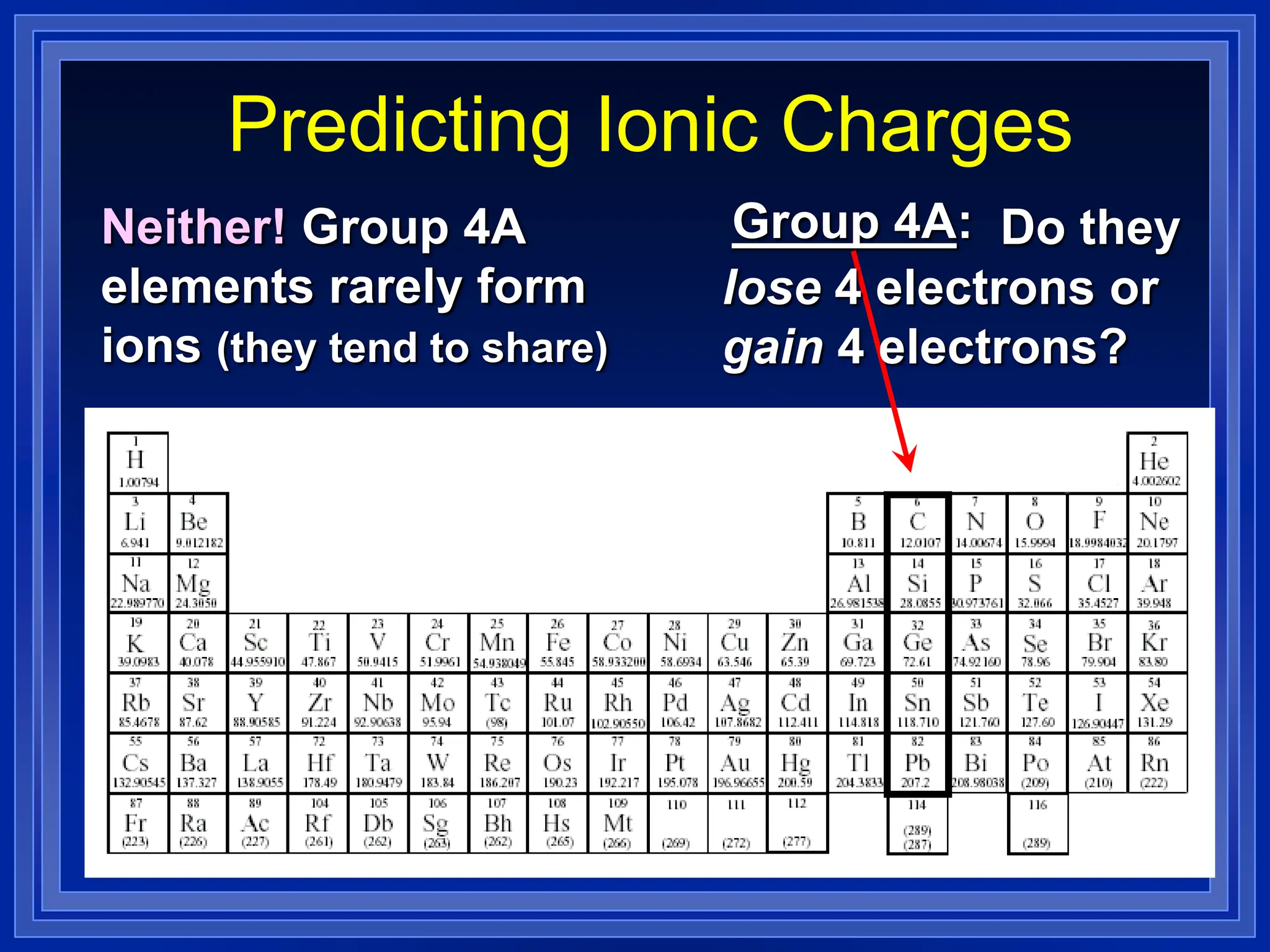
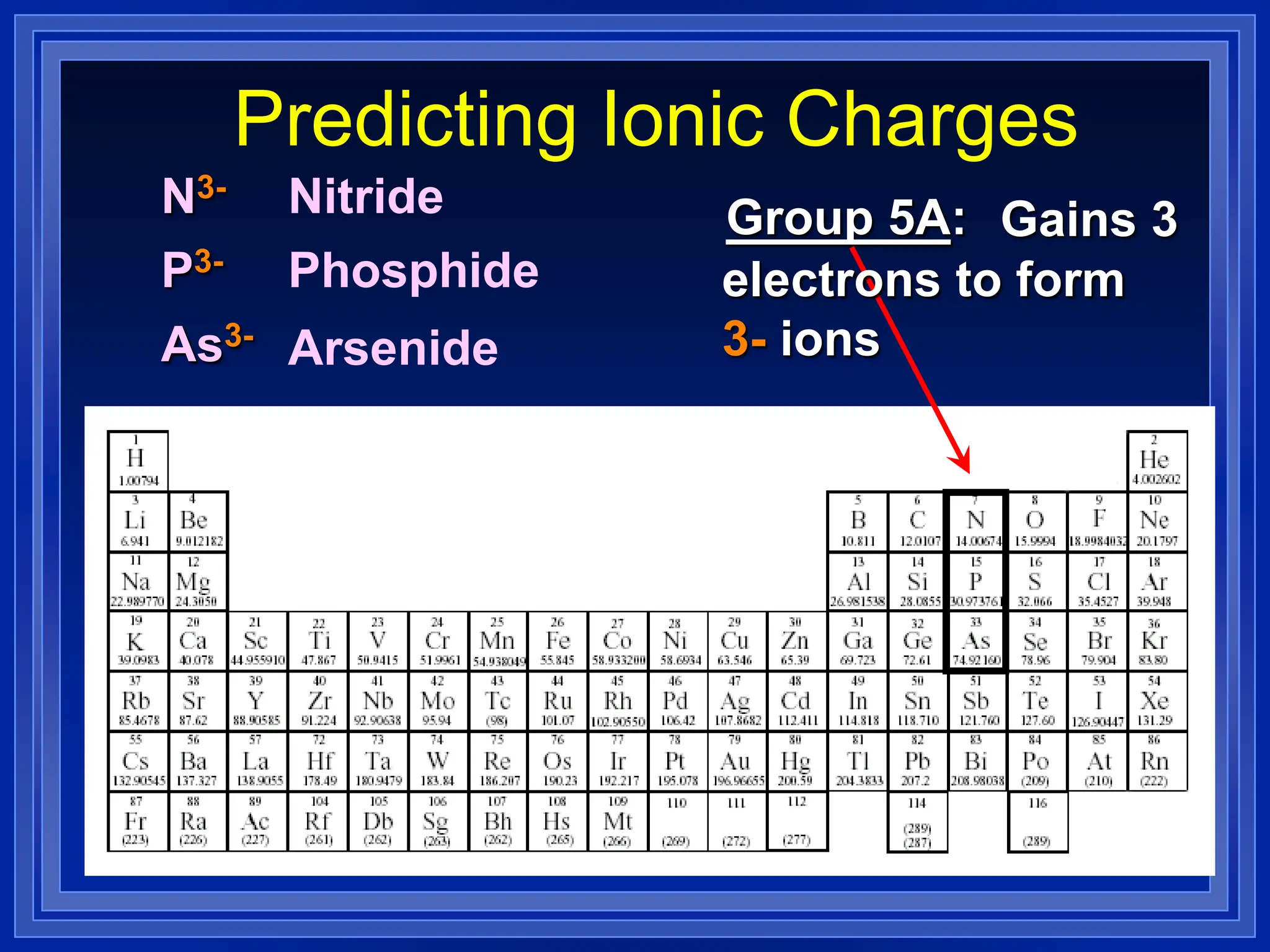
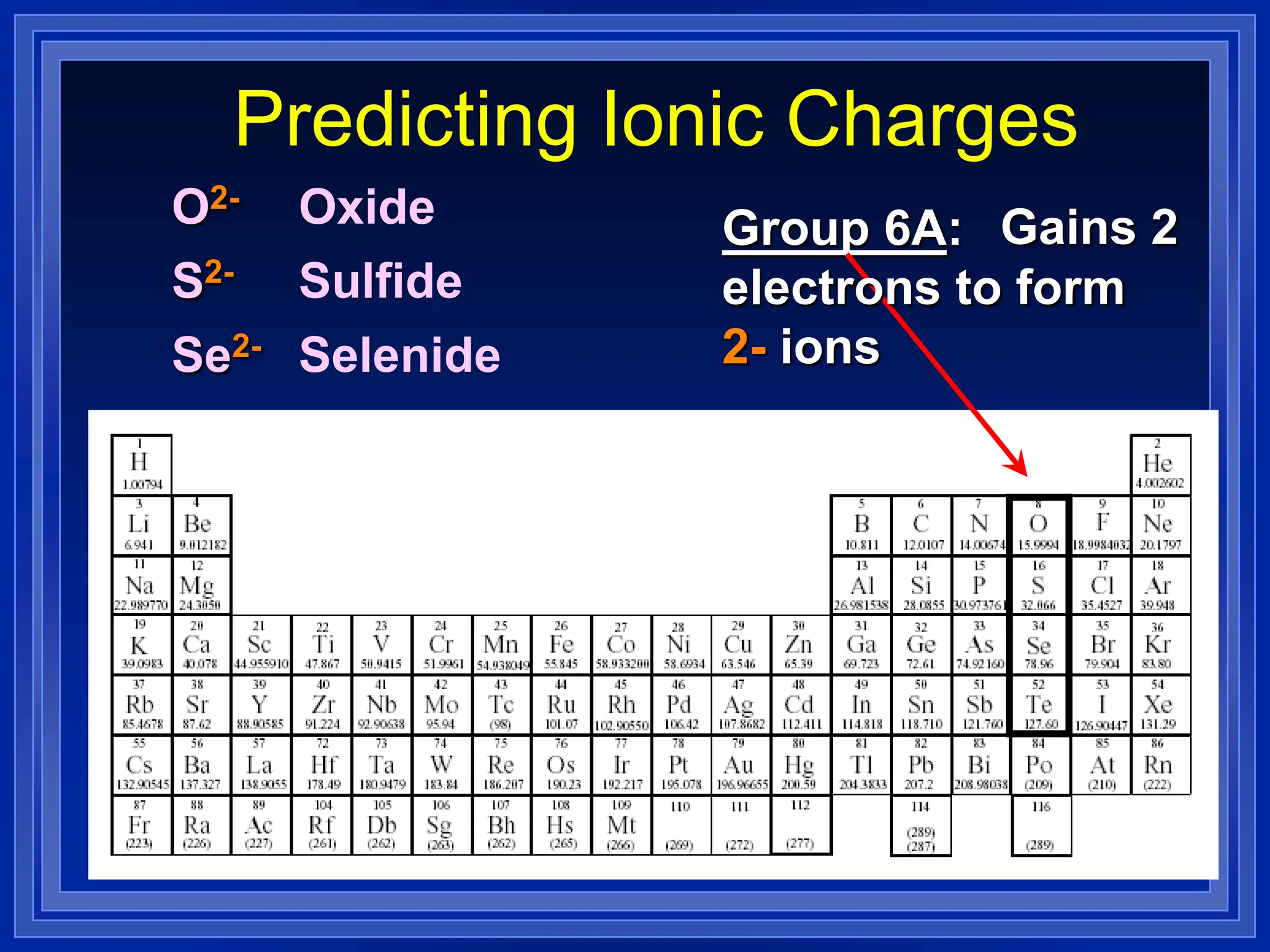
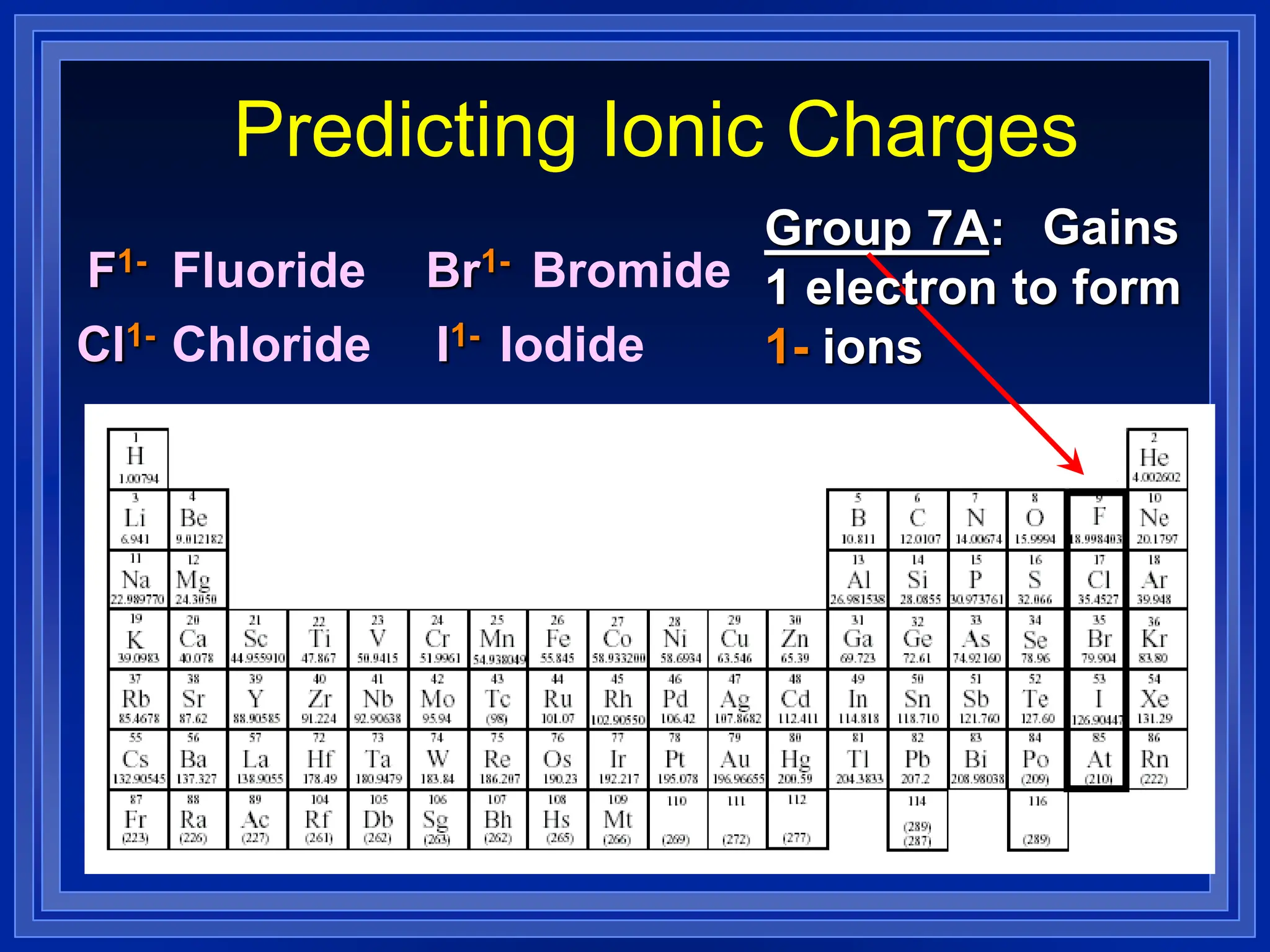
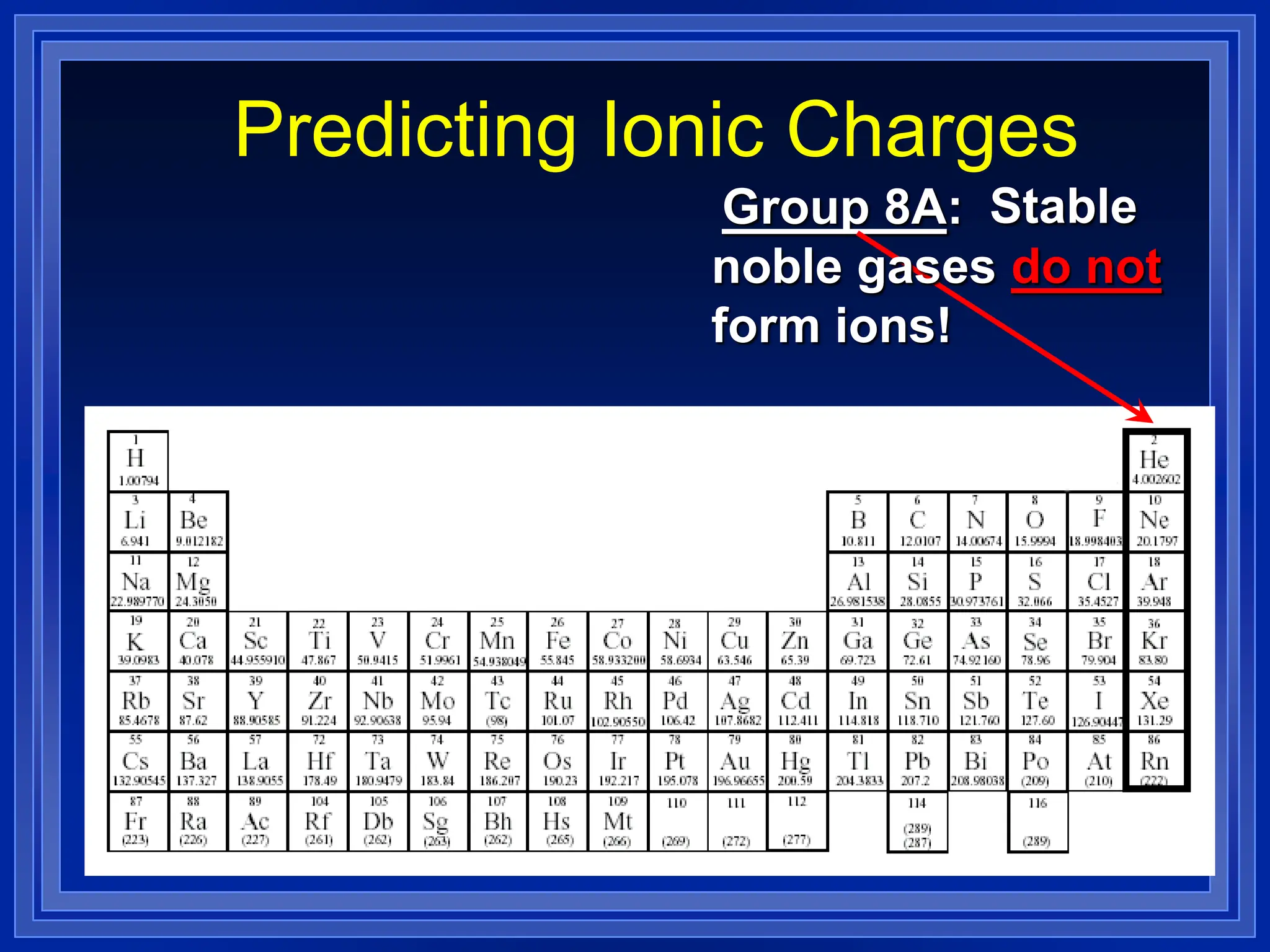
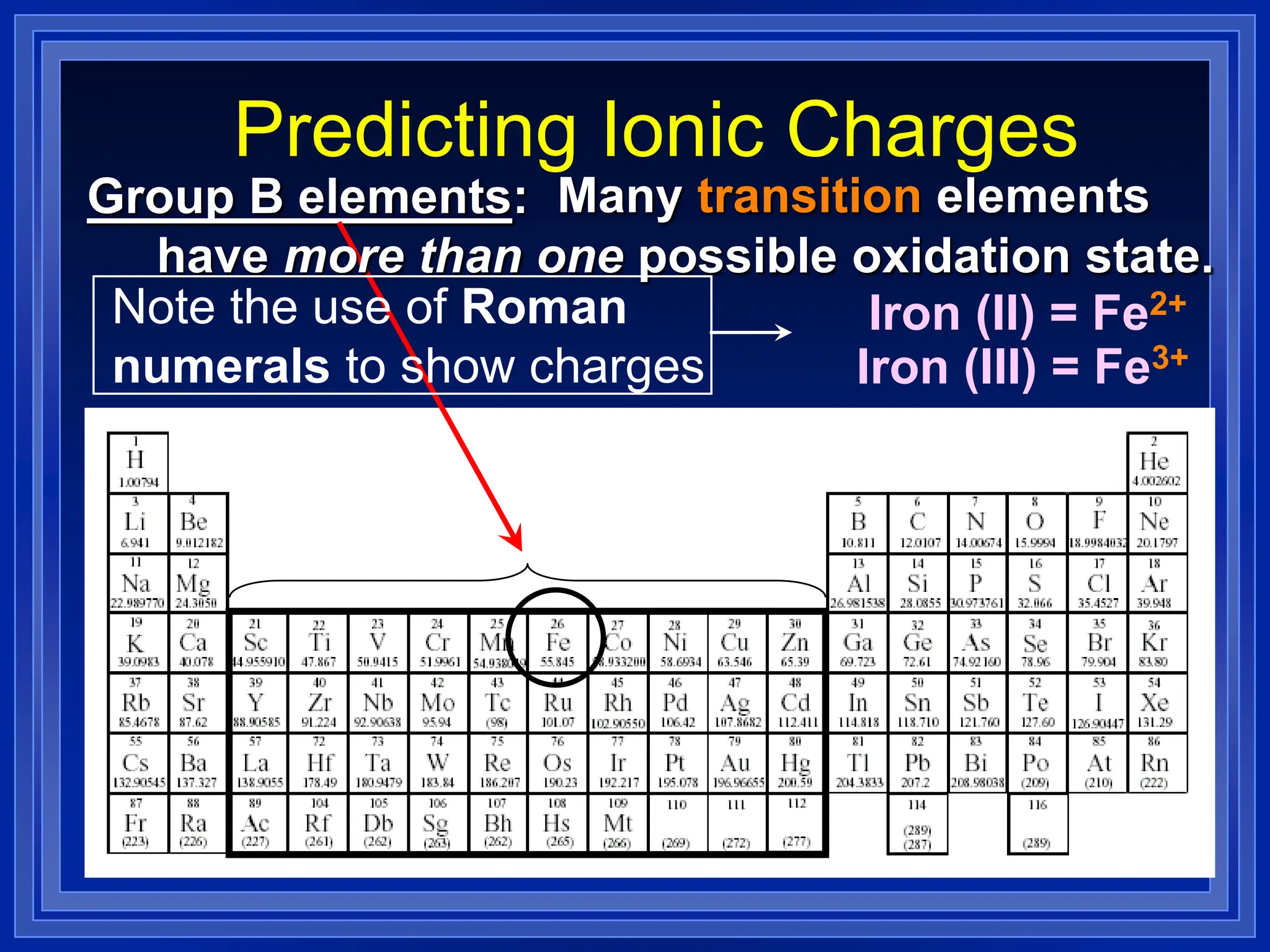
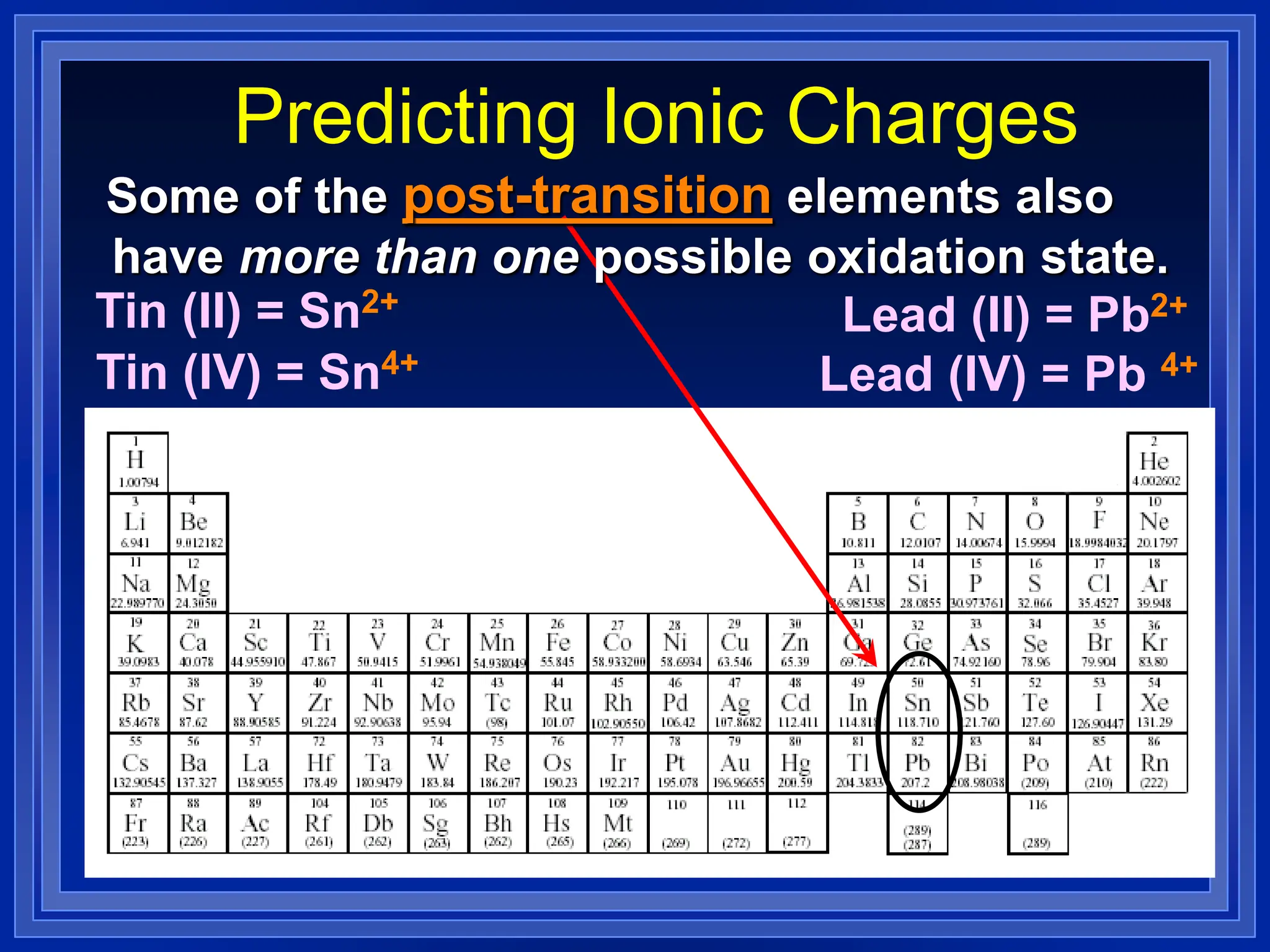
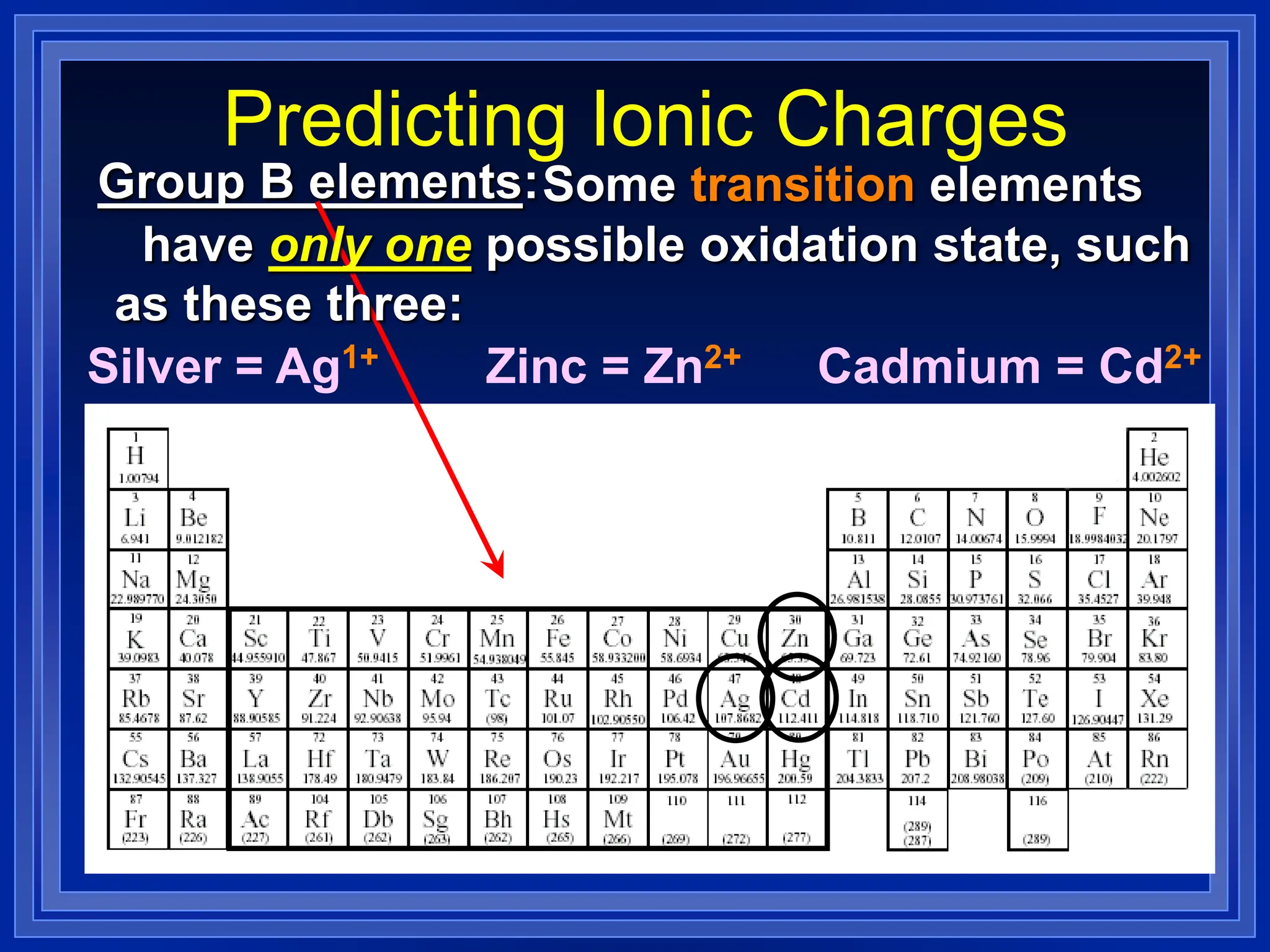
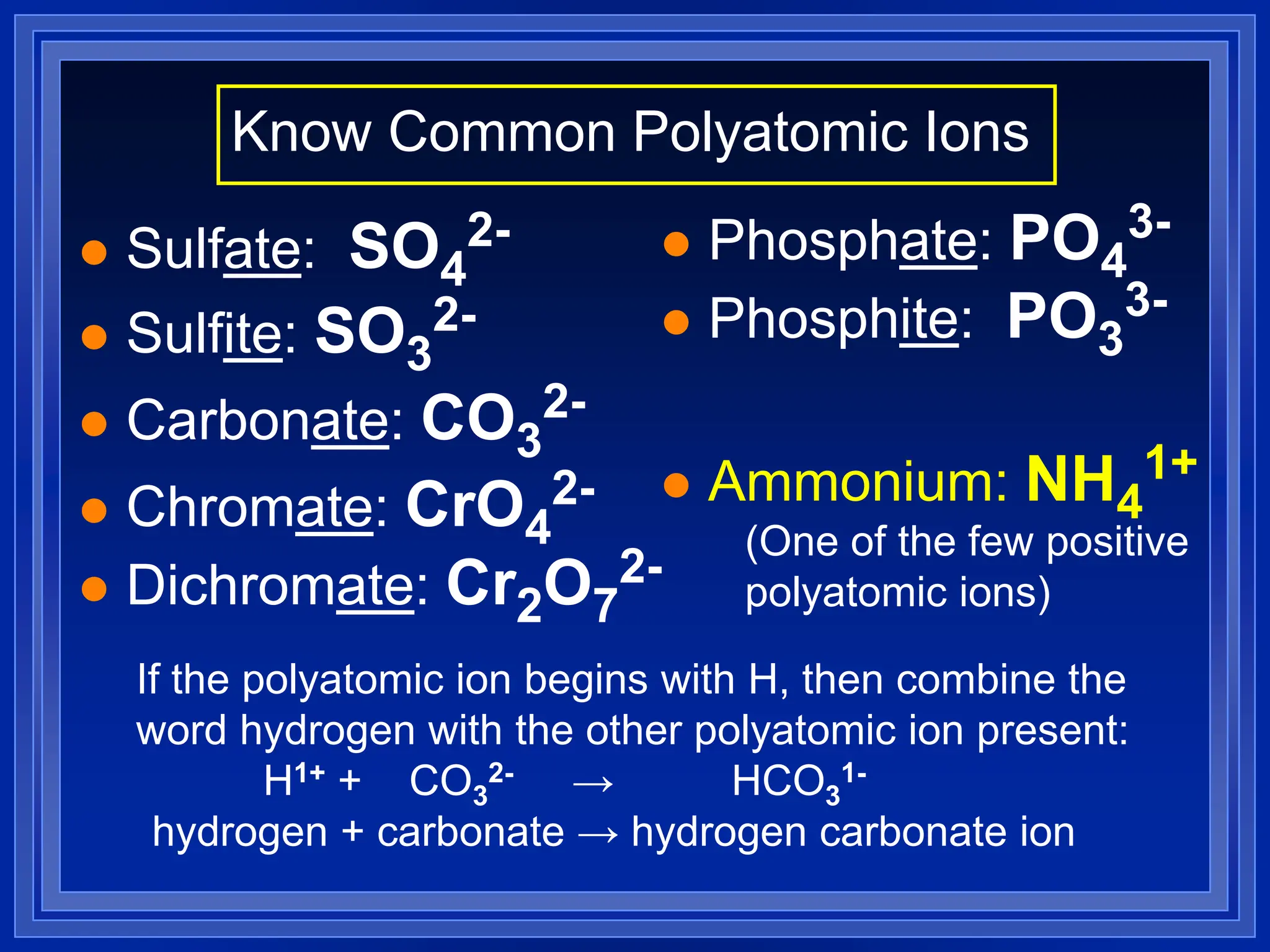
1) Ions and how to predict their charges based on location on the periodic table. Common monatomic and polyatomic ions are identified.
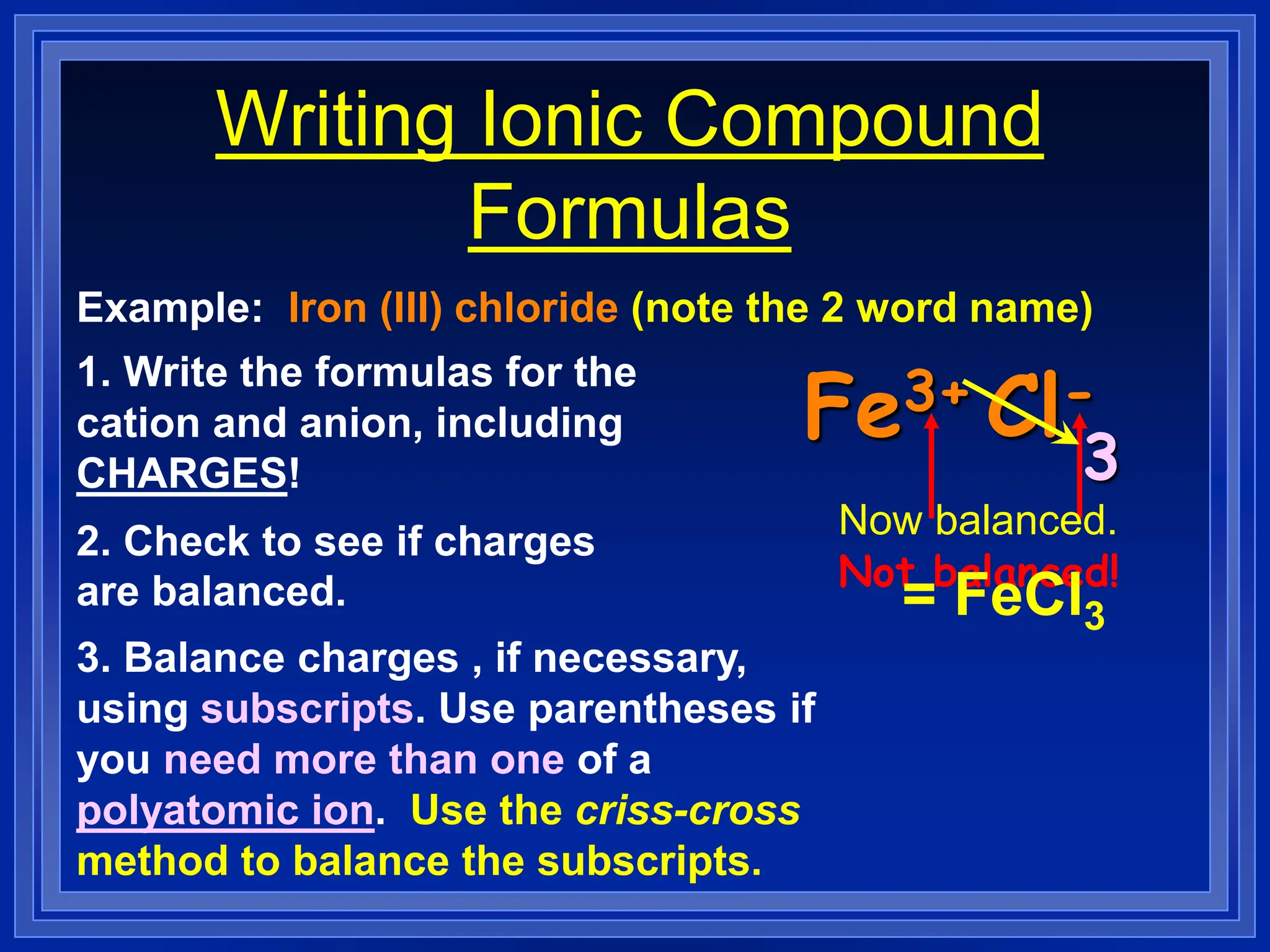
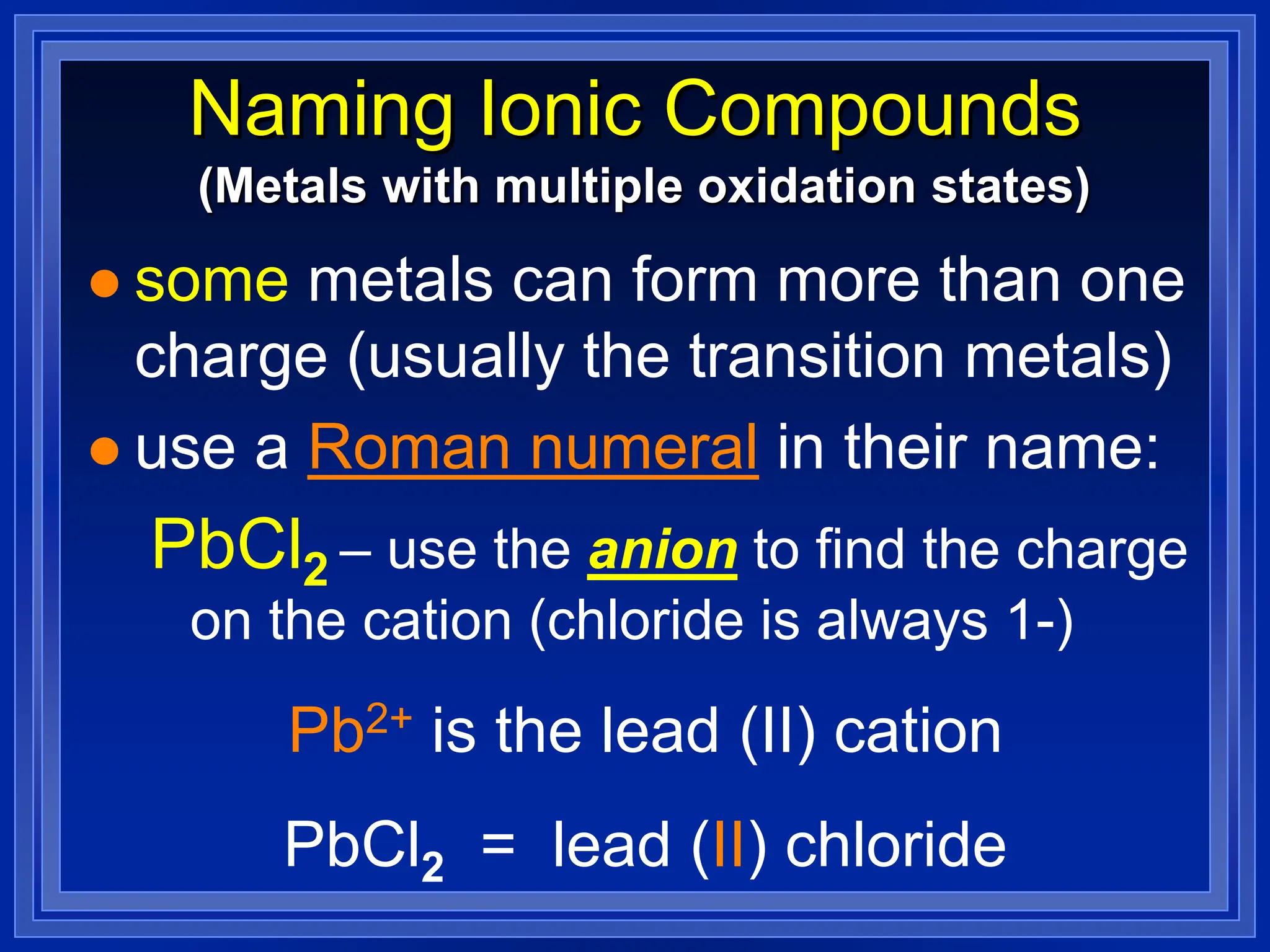
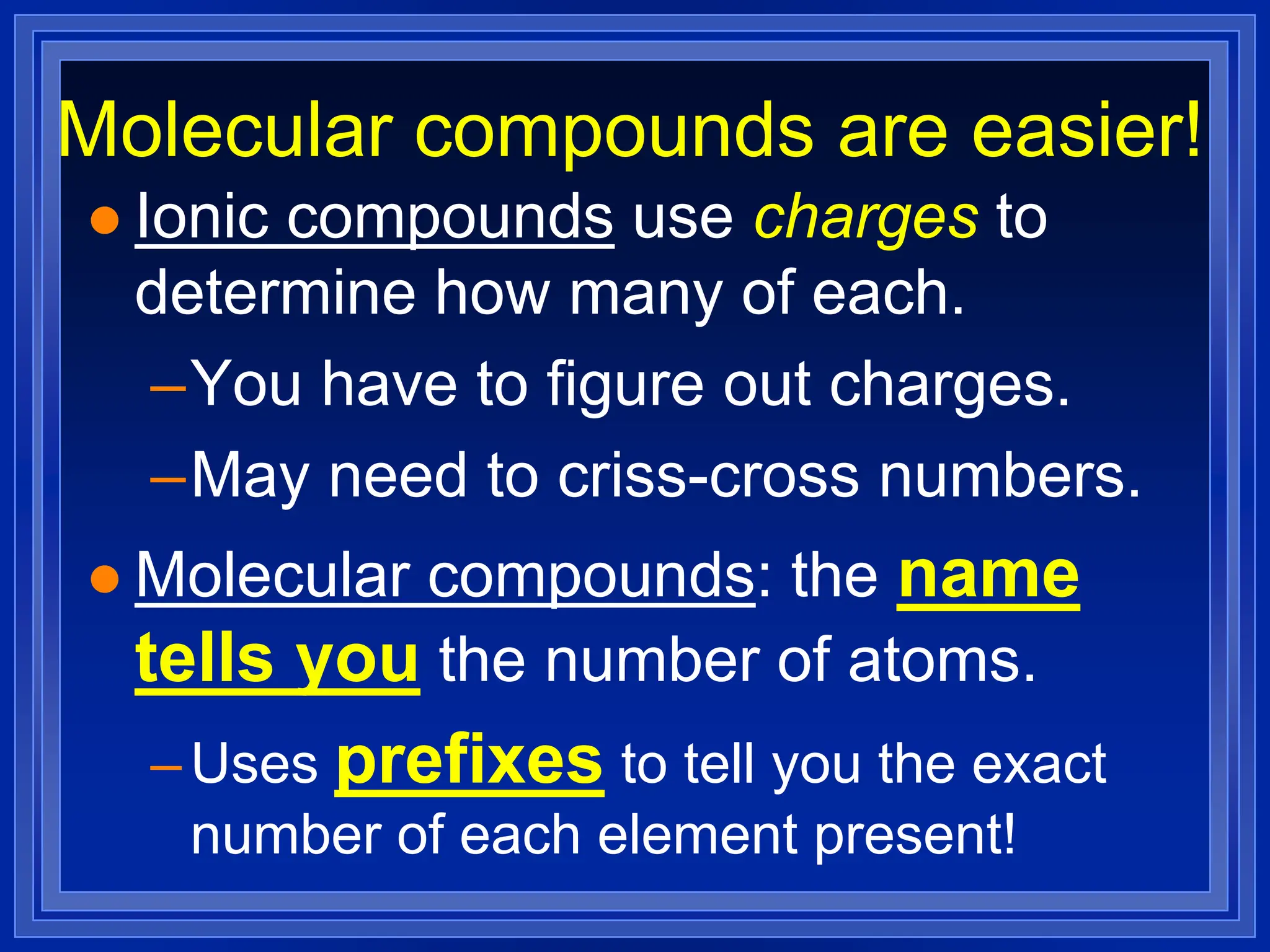
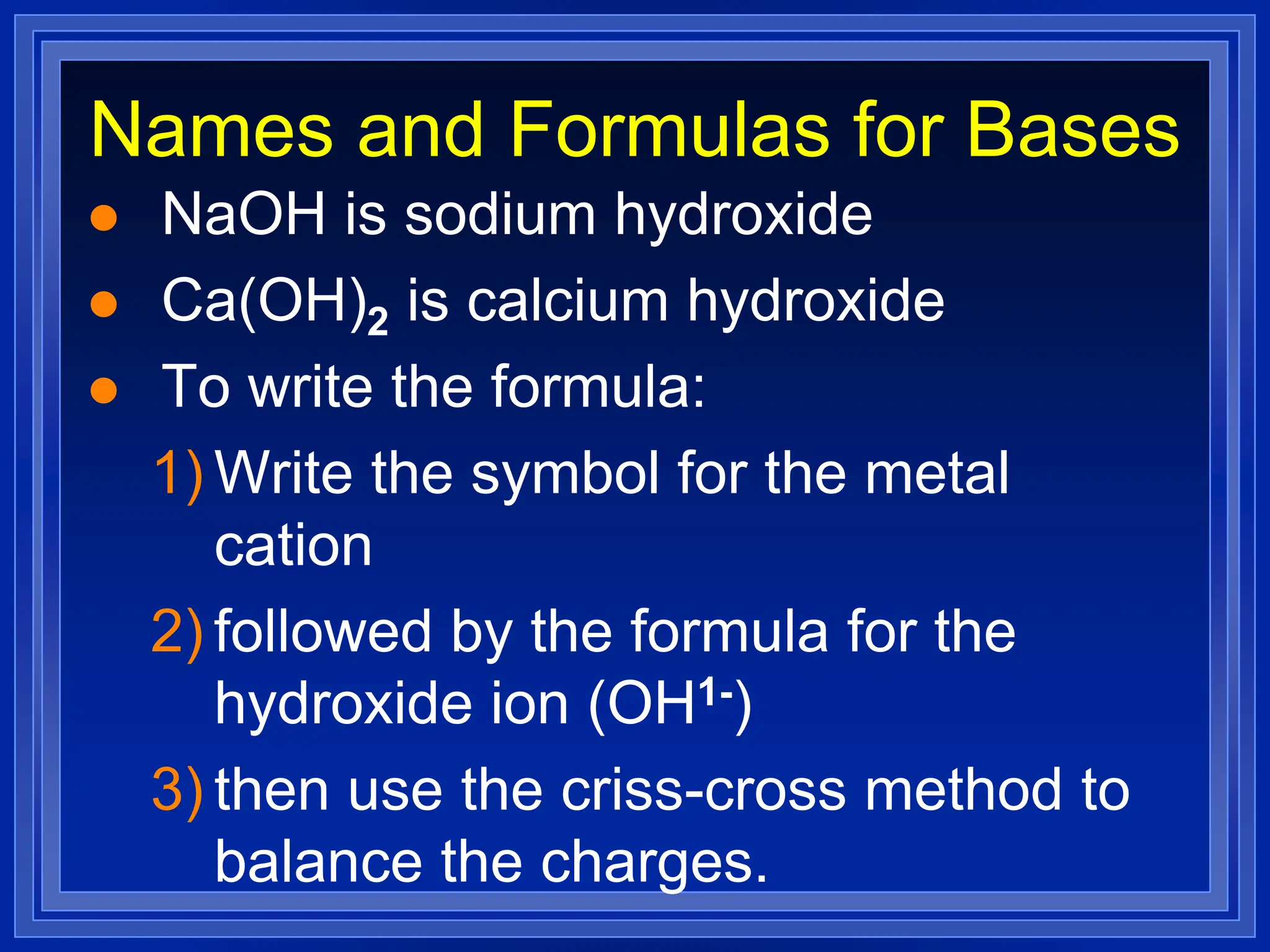
2) Ionic compounds - rules for writing formulas based on cation and anion identities and balancing charges. Rules for naming ionic compounds are also outlined.
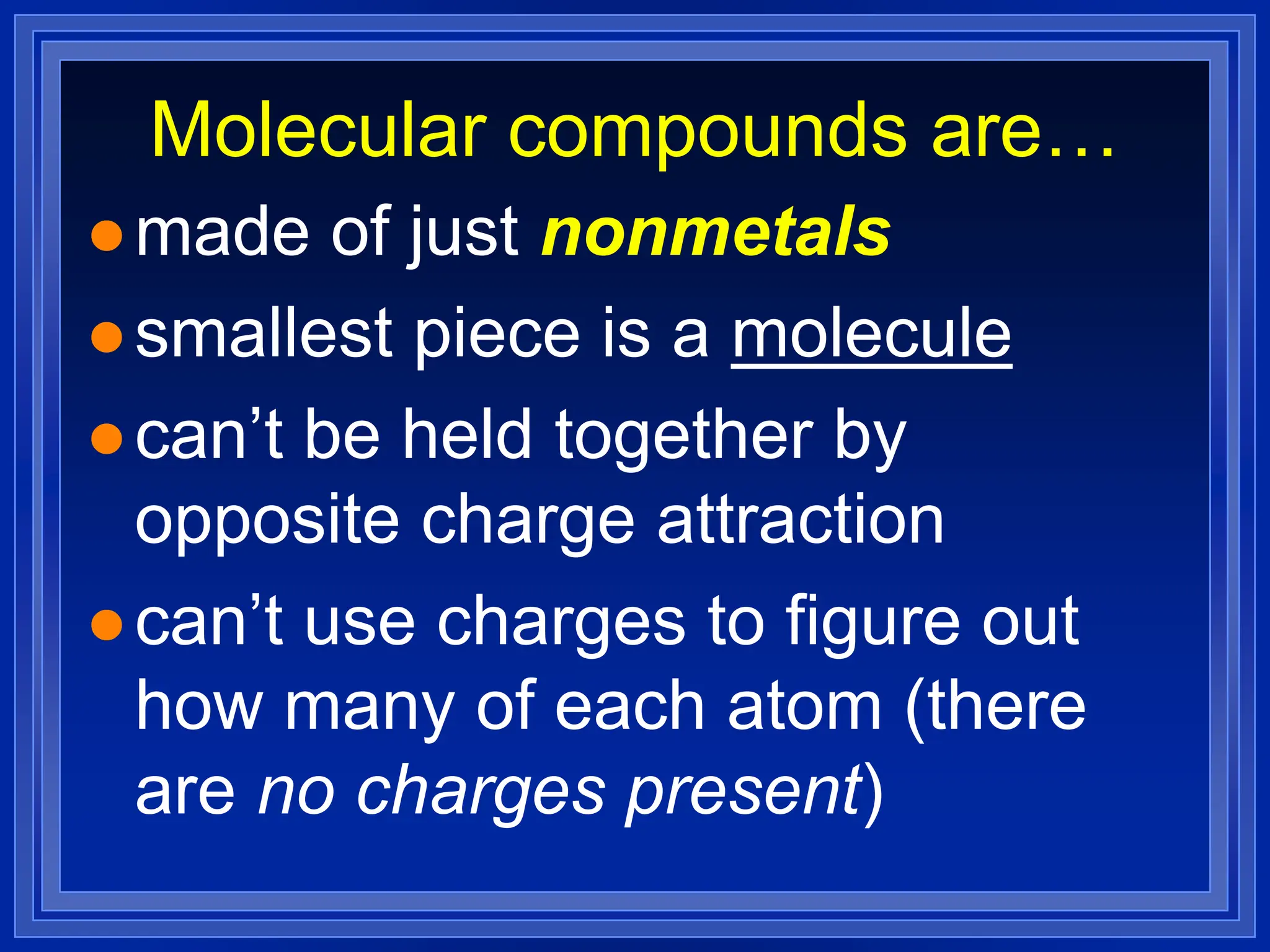
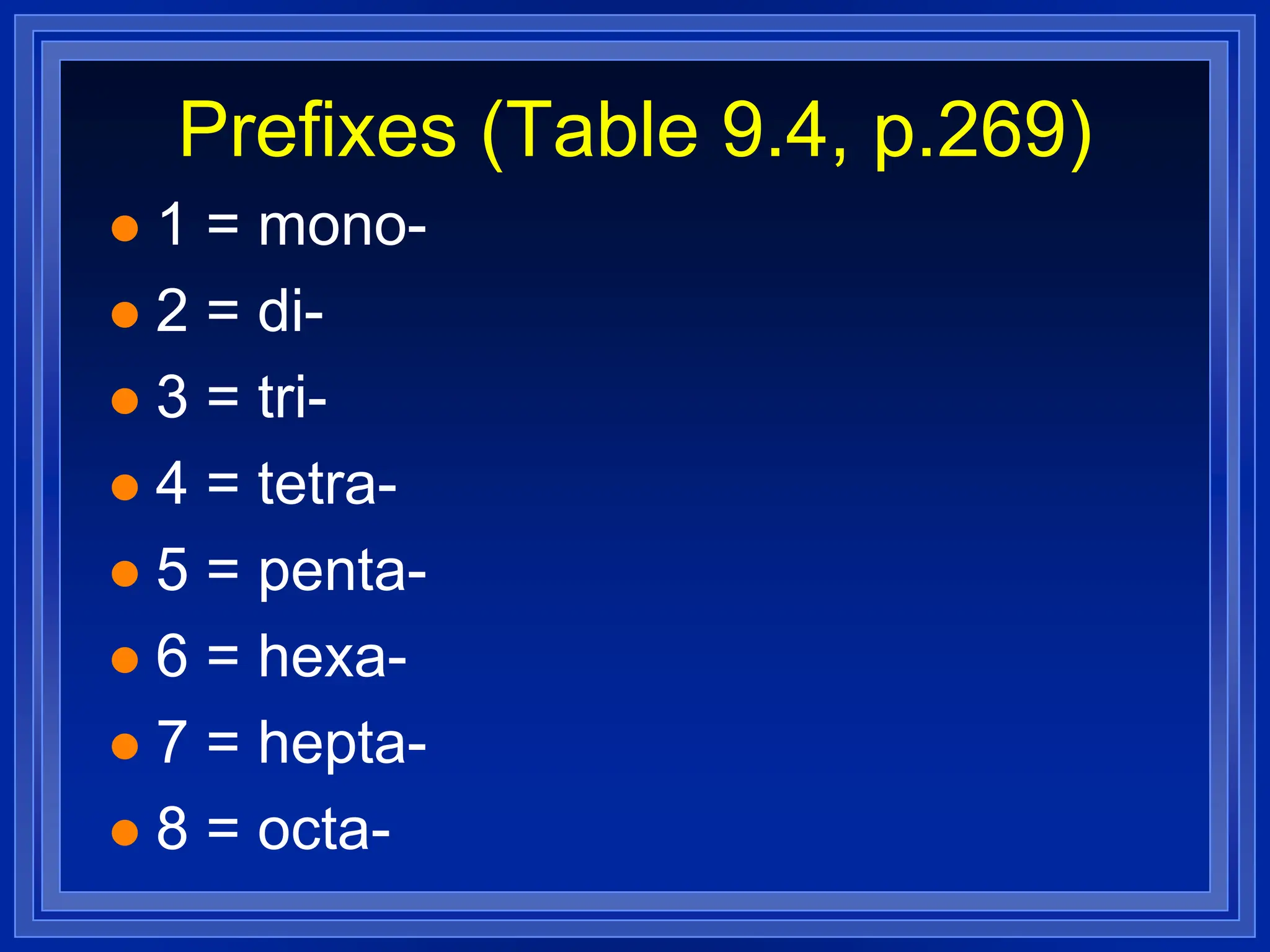
3) Molecular compounds - uses prefixes to indicate number of each atom in the compound name which then directly provides the formula.
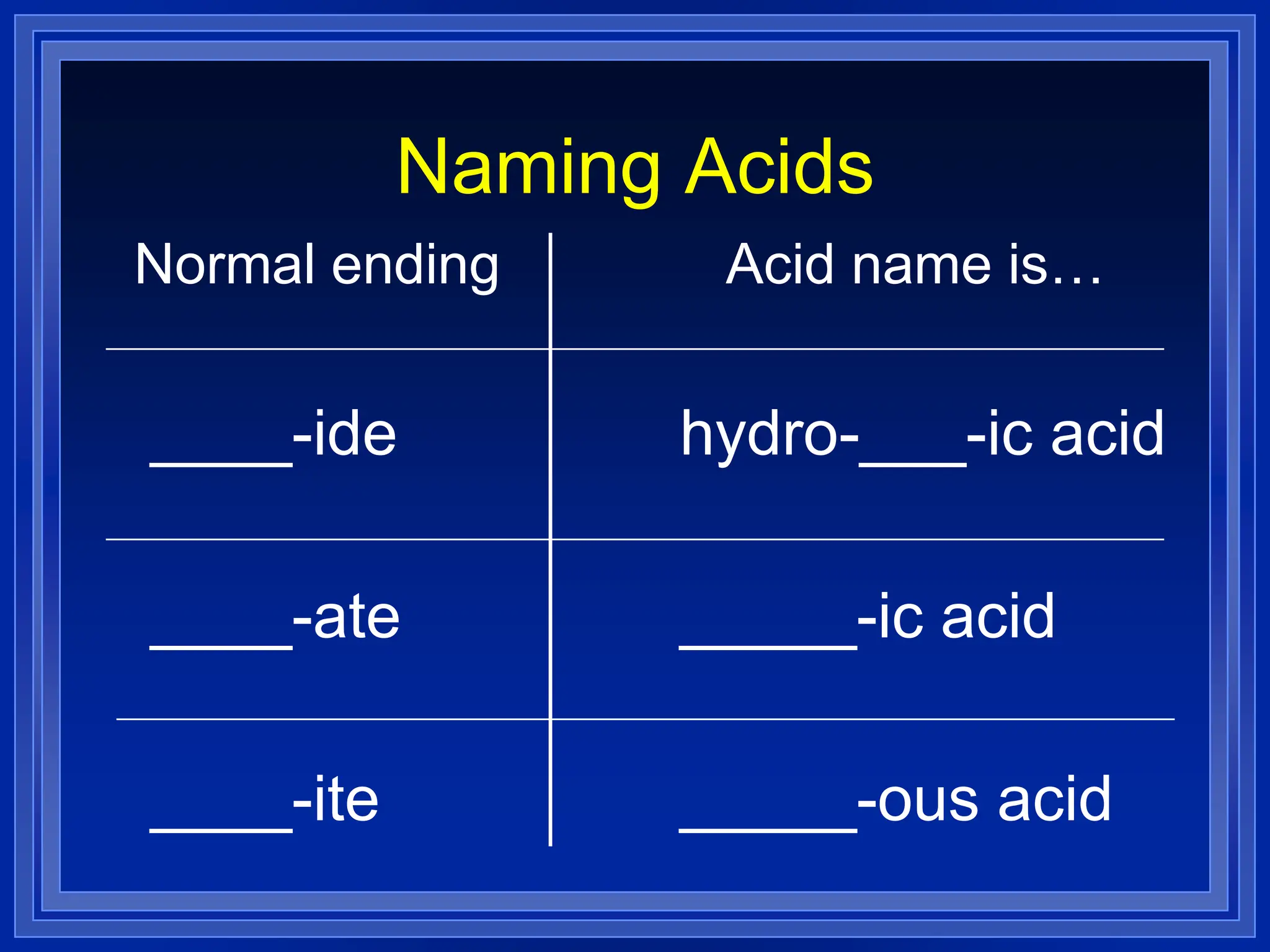
4) Acids - three main rules for identifying if the anion ends in -ide, -ate or -ite and how this determines the acid name and whether a prefix like "hydro