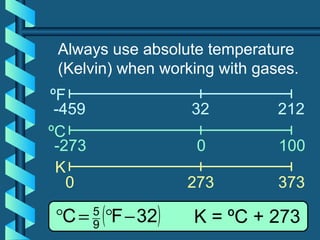
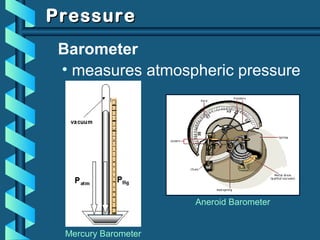
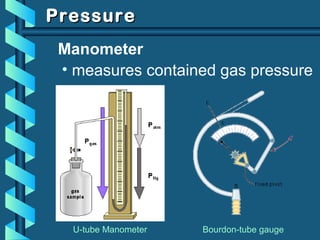
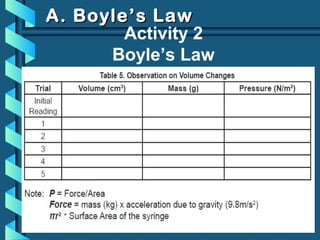
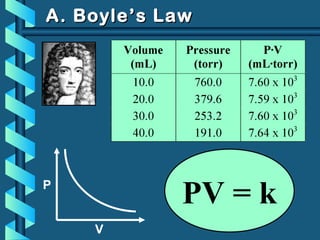
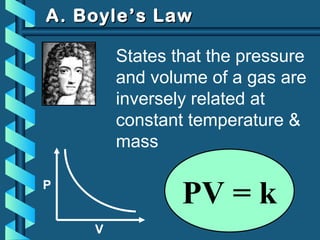
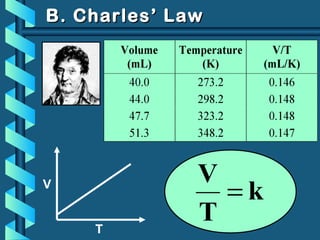
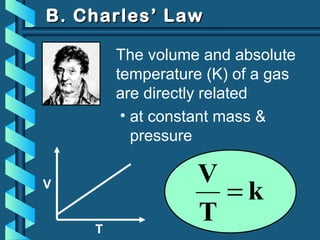
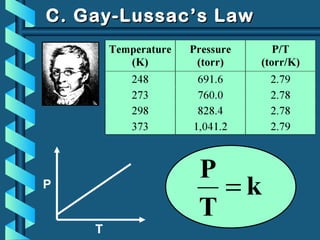
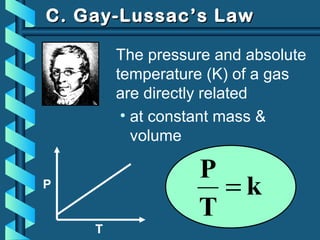
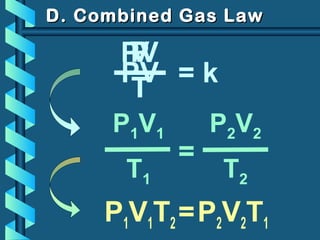
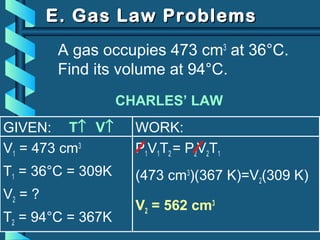
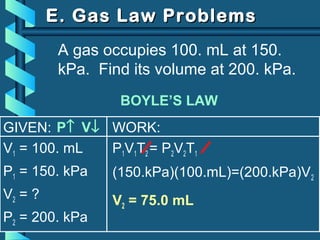
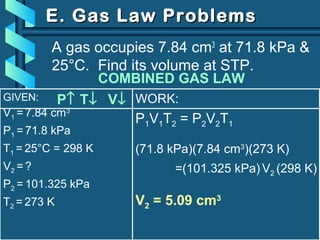
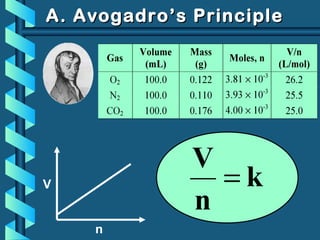
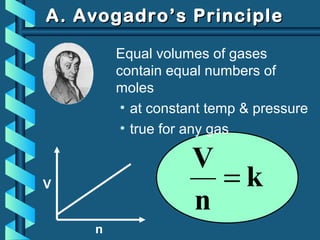
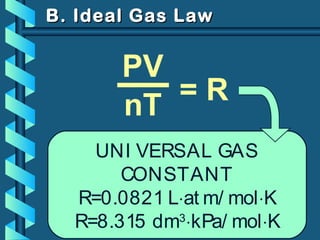
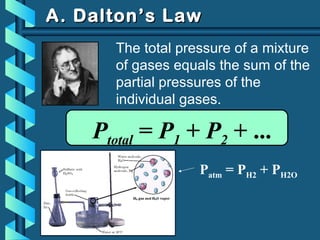
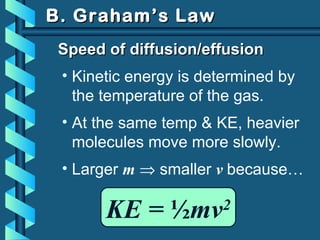
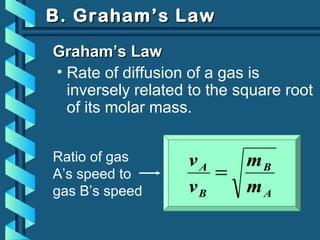
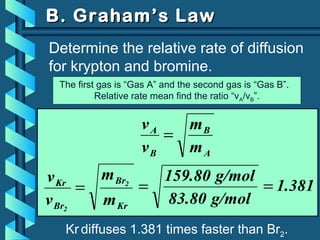
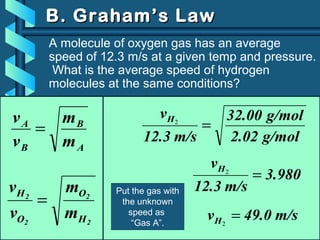
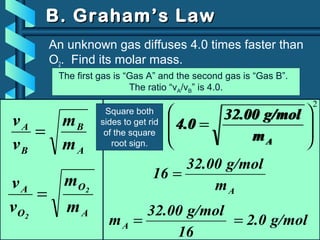
The document provides a comprehensive overview of the properties of gases, including mass, volume, temperature, and pressure, as well as key gas laws like Boyle's, Charles', and Gay-Lussac's laws. It details the relationships between these properties and introduces the ideal gas law along with applications in gas stoichiometry. The document also discusses Dalton's law of partial pressures and Graham's law of diffusion, emphasizing the mathematical relationships and experimental observations related to gases.