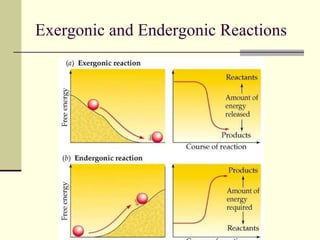
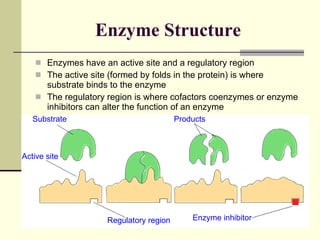
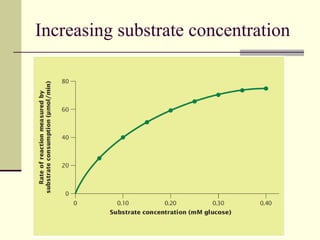
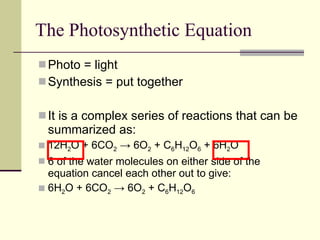
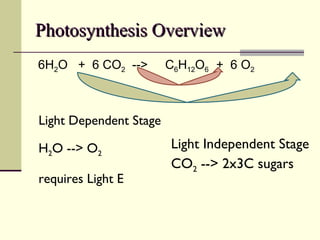
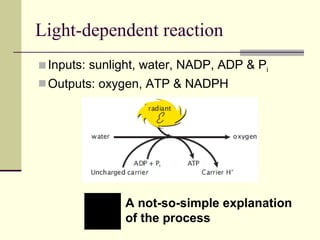
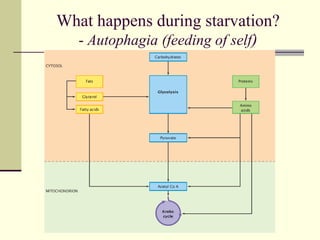
The document discusses energy and cellular respiration in organisms. It explains that organisms obtain energy by breaking down biomolecules through either photosynthesis (autotrophs) or consuming organic matter (heterotrophs). Aerobic respiration fully breaks down glucose to release the most energy as ATP, occurring in three stages: glycolysis, the Krebs cycle in mitochondria, and the electron transport chain. Anaerobic respiration occurs without oxygen and is less efficient, producing lactic acid or ethanol instead of fully oxidizing glucose.