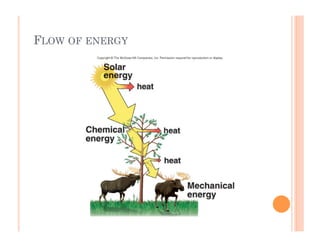
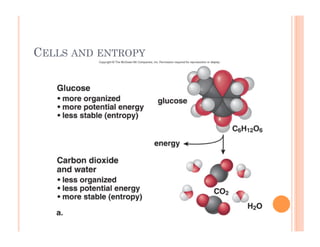
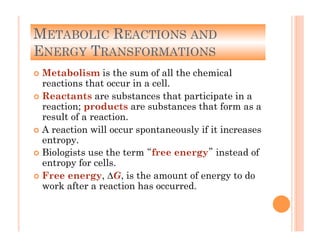
1. Cells require a constant supply of energy to maintain their internal organization and carry out reactions through metabolism. Energy flows through ecosystems in a unidirectional manner as it is transformed and some is lost as heat.
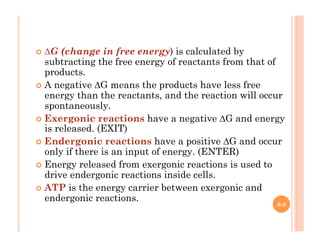
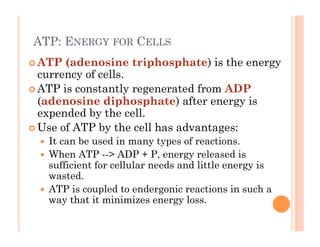
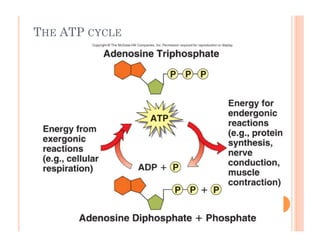
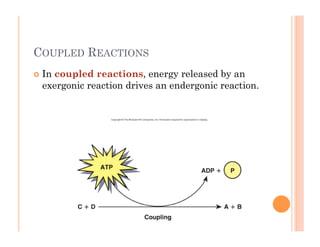
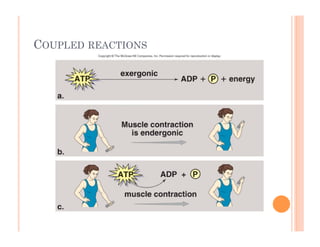
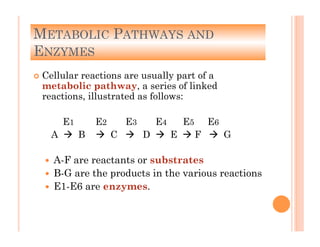
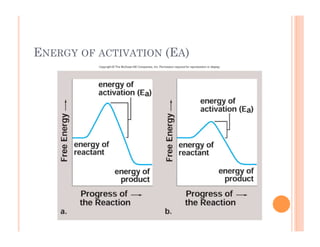
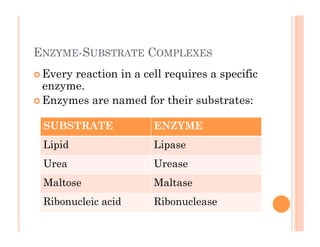
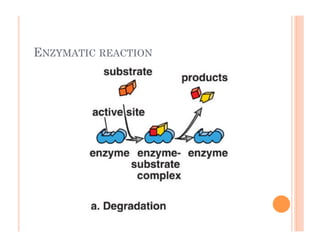
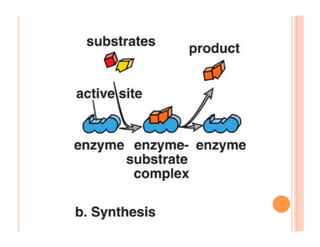
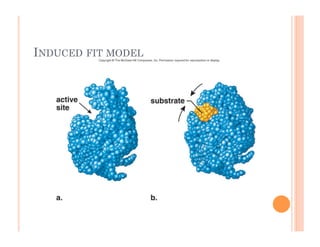
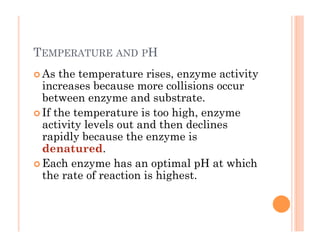
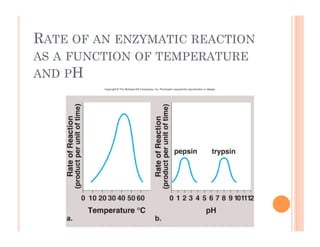
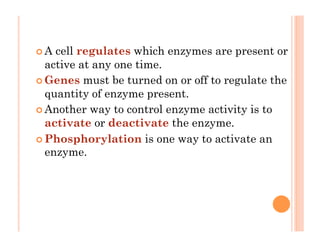
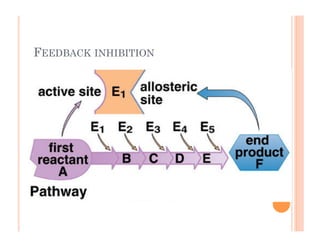
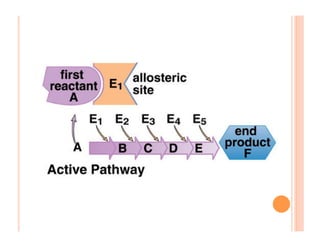
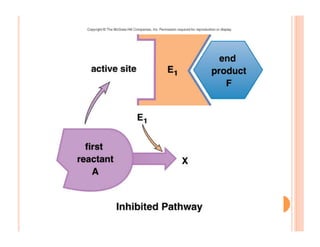
2. ATP is the energy currency of cells that is regenerated through exergonic reactions and used to drive endergonic reactions. Metabolic pathways involve series of enzyme-catalyzed reactions that convert food into ATP.
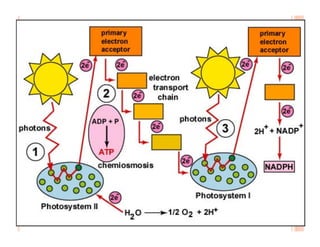
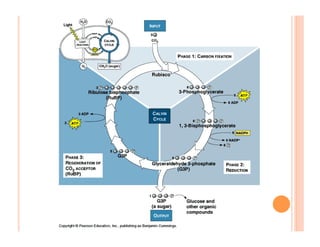
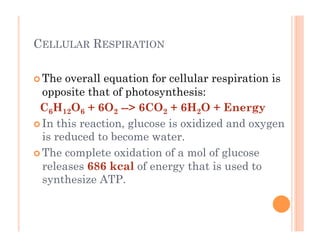
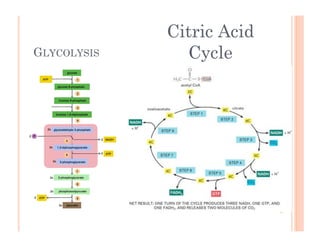
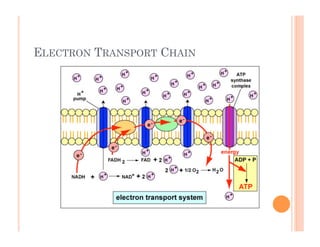
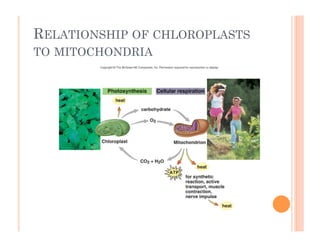
3. Photosynthesis and cellular respiration are key redox reactions that allow the flow of energy from the sun through living things by cycling molecules between chloroplasts and mitochondria.