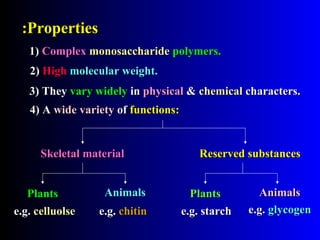
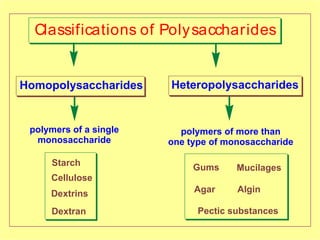
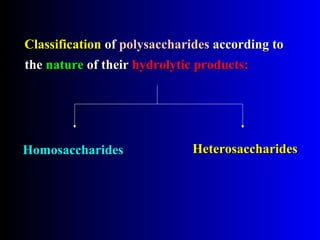
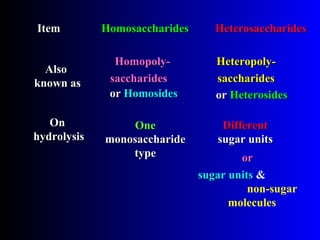
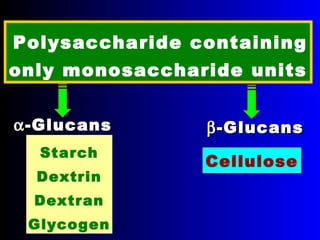
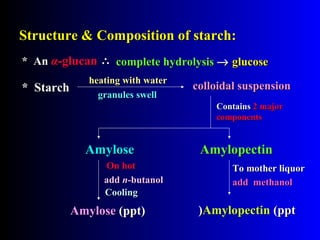
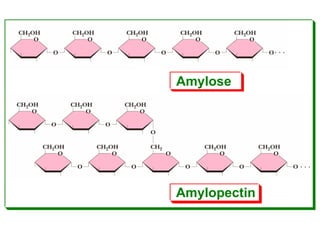
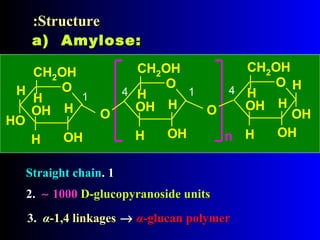
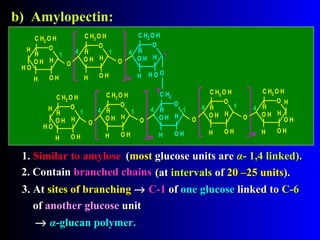
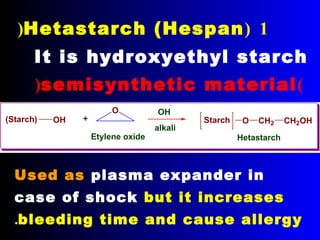
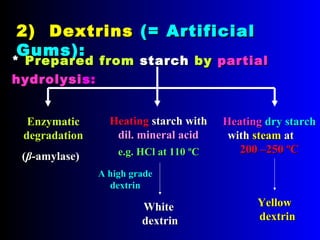
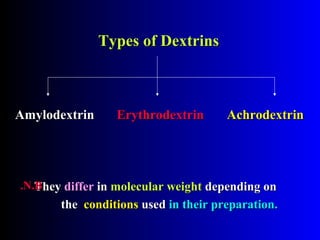
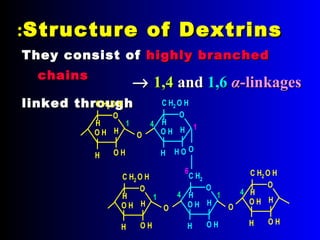
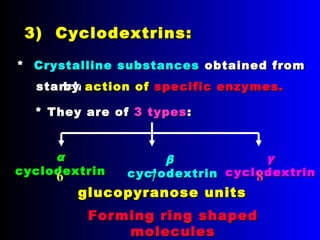
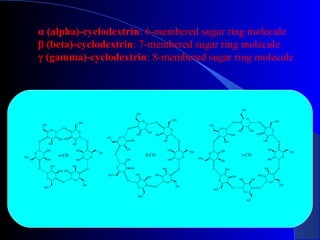
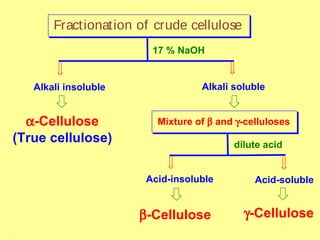
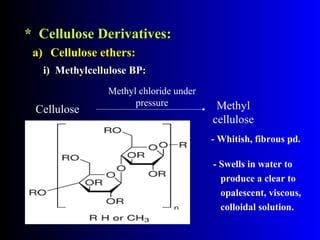
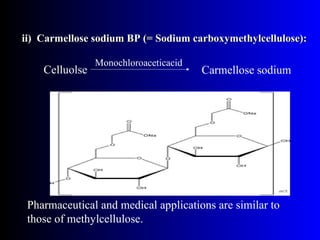
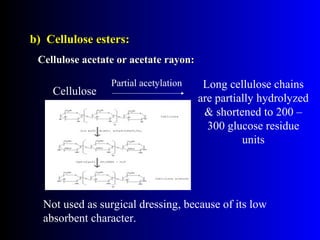
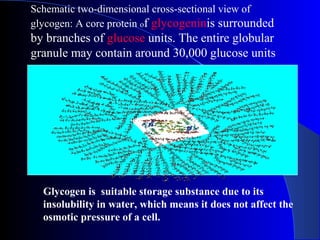
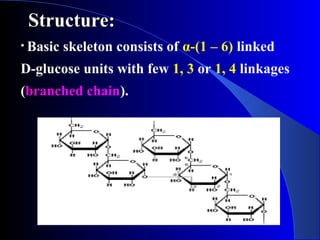
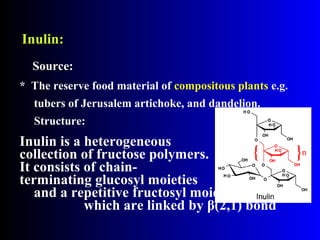
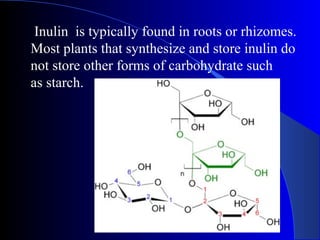
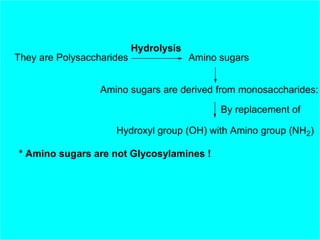
Polysaccharides are complex monosaccharide polymers that serve a wide variety of functions. They can be classified as homopolymers containing a single monosaccharide unit or heteropolymers containing different sugar units. Starch is a major plant polysaccharide composed of amylose and amylopectin. It is used as food, in pharmaceuticals, and to produce dextrins and soluble starch. Dextrins are prepared from starch by partial hydrolysis and are used as substitutes for gums. Cyclodextrins are obtained from starch and have a hydrophobic central cavity, making them useful for enclosing drugs.


















































![O
HO
HO
NHCOCH3
O
OH
O
OH
NHCOCH3
OH
CH2OH
O
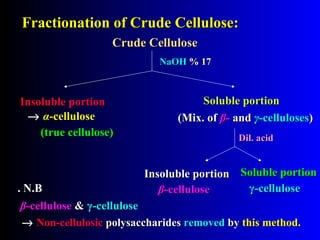
1) HCl
2) wash with H2O
3) dil. NaOH (deproteination)
CaCO3 & proteins are removed
1) wash with H2O
2) wash with organic solvent
(to remove pigments,[ carotenoids])
3) dry
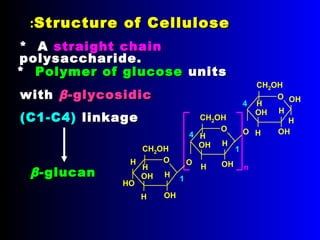
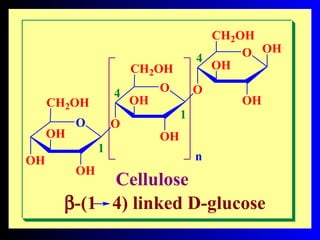
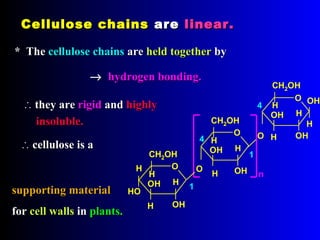
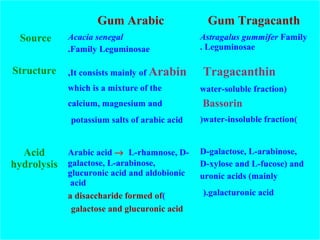
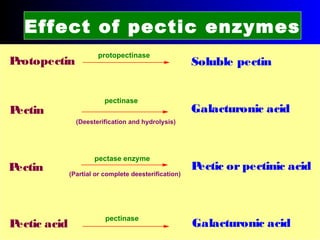
Chitin is a linear polymer of chitobiose
The most important sources of chitin are the large amounts of waste crab
and krill shells from the fishing industry,
Waste products of crab shells are decalcified by
Production of chitin
2
O
NHCOCH3
OH
CH2OH
OH
4
1
O
HO
NHCOCH3
OH
OH
Chitobiose
2-acetamido-2-dioxy-D-glucose Chitin
2 molecules of N-acetyl-D- glucosamine with β-(1-4) linkage
Acid hydrolysis
Enzymatic hydrolysis
Acetic acid + D-glucosamine (= 2-aminoglucose)
N-acetyl-D-glucosamine](https://image.slidesharecdn.com/polysaccharidesazhar-151130204500-lva1-app6892/85/Polysaccharides-85-320.jpg)







