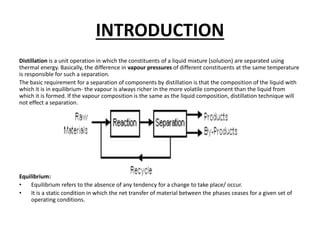
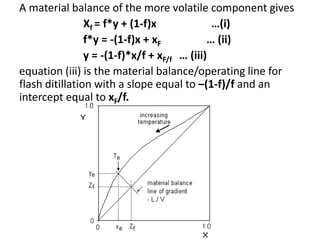
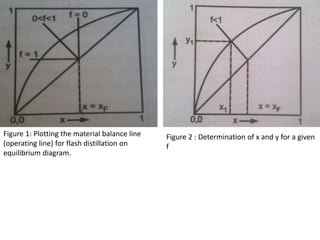
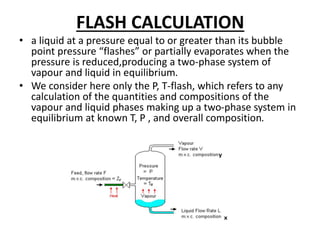
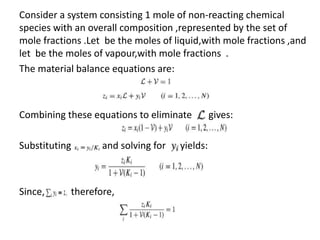
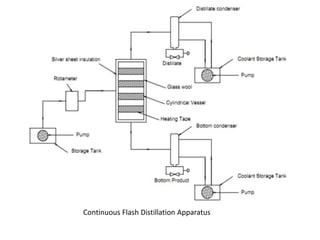
The document describes continuous flash distillation. Flash distillation involves partially vaporizing a liquid mixture, allowing the vapor and liquid to reach equilibrium, and then withdrawing them separately. Material balances are used to model flash distillation. The flash distillation process is commonly used in the petroleum industry to separate petroleum fractions by heating the fluid and "flashing" it into an overheated vapor stream and residual liquid stream.

![ABSTRACT
Distillation is a method of separating mixtures based on differences
in volatilities of components in a boiling liquid mixture.
Distillation is basically carried out in three ways:
1) Differential or simple distillation.
2).Rectification or fractionation.
3)Flash or equilibrium distillation.
The Flash Distillation model is normally carried out either
continuously or in batches. In this method, a liquid mixture is partially
vaporized, the vapor and liquid are allowed to attain equilibrium and
finally withdrawn separately.
Material balance in a Flash distillation column is -
[accumulation] = [in] - [out] + [generation] - [consumption]
The flash distillation is used on a large scale in petroleum industry in
which petroleum sections are heated in pipe stills and the heated fluid
flashed into overheated vapor and residual liquid streams](https://image.slidesharecdn.com/flashdistillation-160119140500/85/Flash-distillation-2-320.jpg)