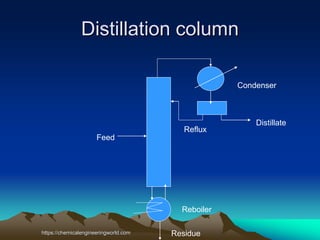
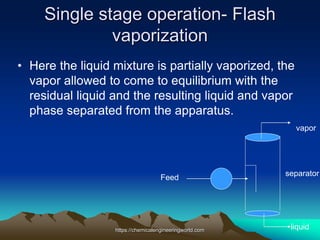
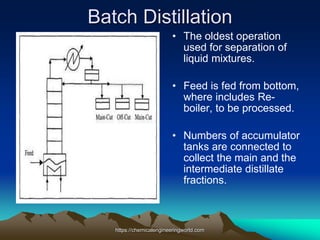
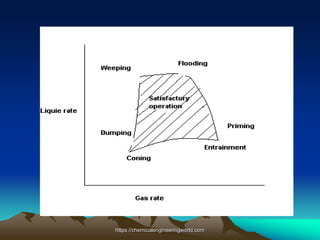
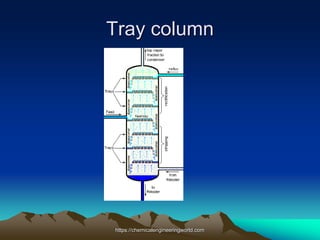
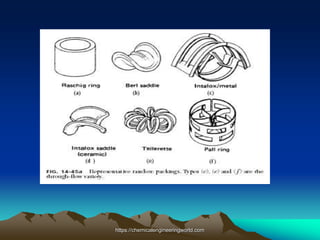
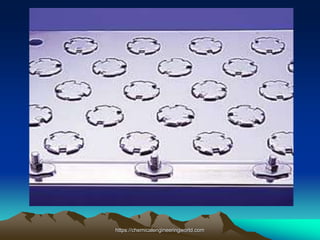
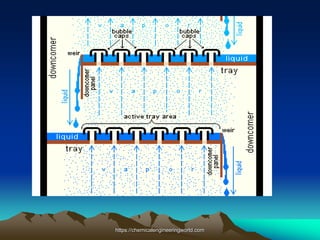
The document provides an overview of distillation, including its definition, methods, and key parameters such as reflux ratio and relative volatility. It compares distillation with other mass transfer operations and discusses various types of distillation processes, along with potential problems encountered in tray and packed towers. Additionally, it highlights the differences between tray and packed columns in industrial applications.