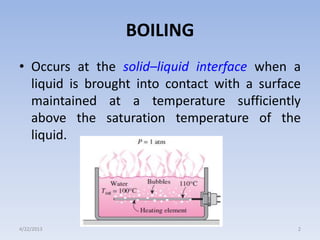
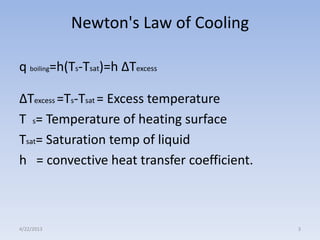
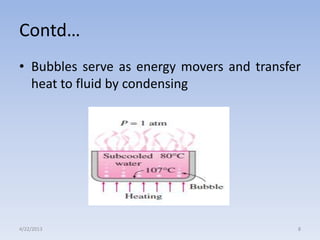
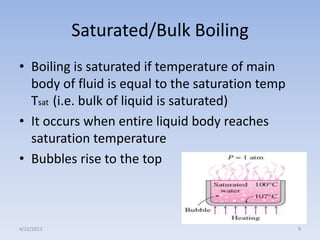
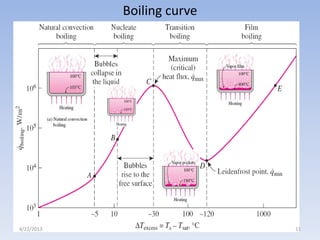
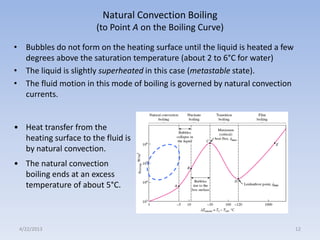
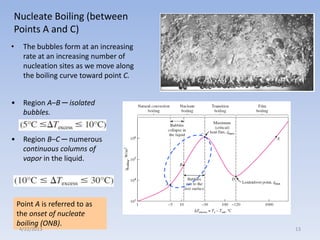
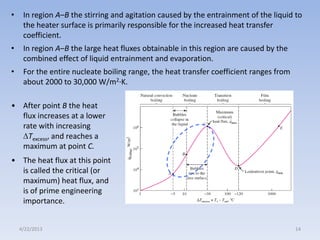
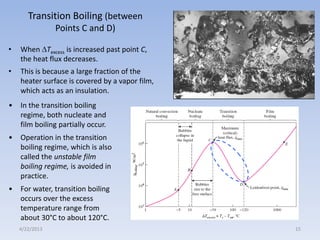
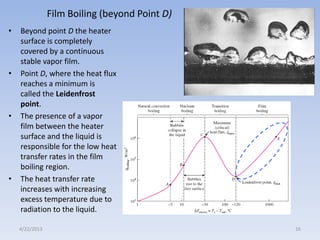
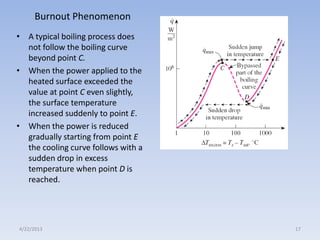
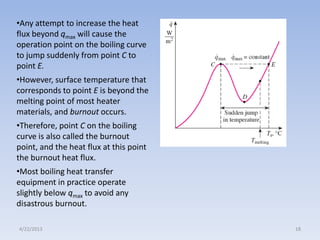
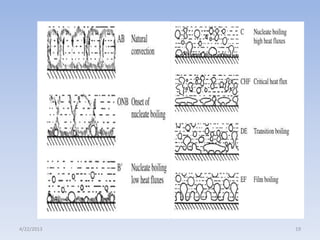
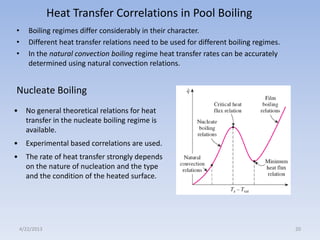
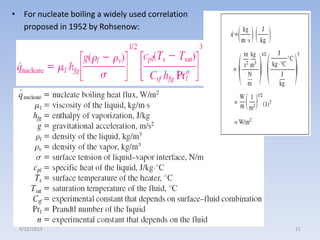
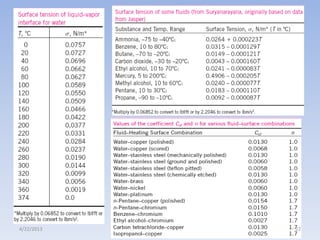
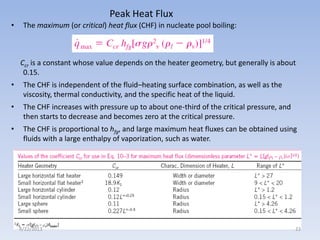
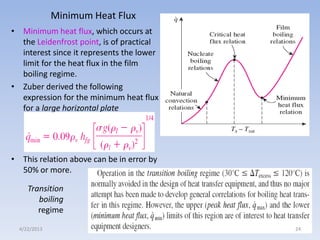
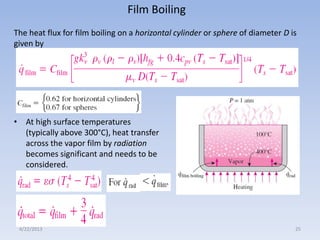
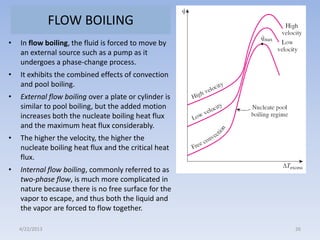
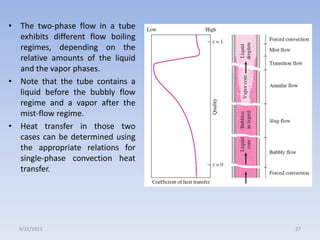
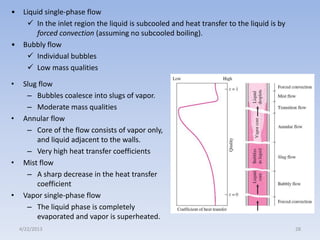
The document analyzes boiling curves and forced convection boiling. It discusses pool boiling and flow boiling, subcooled and saturated boiling, and the various regimes that occur on the boiling curve - natural convection, nucleate boiling, transition boiling, and film boiling. Heat transfer correlations are presented for different boiling regimes, including the Rohsenow correlation for nucleate boiling and relationships for critical heat flux and minimum heat flux. Flow boiling is also examined, describing different flow patterns that can occur in two-phase flow inside a tube.