Embed presentation
Downloaded 41 times
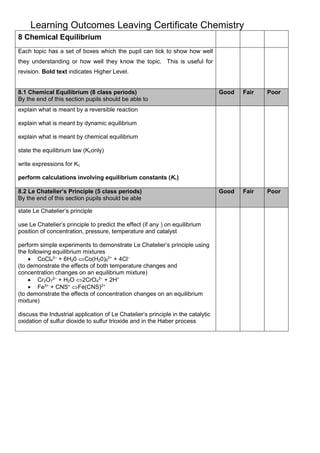
This document outlines the key learning outcomes for two topics in Leaving Certificate Chemistry: 8.1 Chemical Equilibrium and 8.2 Le Chatelier's Principle. For 8.1, students should be able to explain concepts related to reversible reactions and chemical equilibrium, write equilibrium constant expressions, and perform calculations involving equilibrium constants. For 8.2, students should be able to state Le Chatelier's principle and use it to predict how concentration, pressure, temperature, and catalysts affect equilibrium position, as demonstrated through experiments on example chemical mixtures. Industrial applications are also discussed.
