This document provides background information on reaction rates and mechanisms. It discusses how factors like reactant concentrations, temperature, catalysts, and surface area can influence reaction rates. It also defines concepts like the rate law, rate constant, reaction order, energy of activation, and Arrhenius equation. Methods for determining reaction order are described, including by varying reactant concentrations and analyzing integrated rate expressions for zero, first, and second order reactions. The effects of temperature on reaction rates are also addressed through the Arrhenius equation.

![II. Rates
Reaction Rate = either the increase in M of product per unit time
or the decrease in M of reactant per unit time; ΔM / ΔT
Note: [X] = moles X / Liter
Example: H+ Catalyst
Sucrose + H2O --------------) Glucose + Fructose
Rate = rate of formation of either product.
Rate = Δ M of glucose / Δsec = Δ[glucose] / Δsec
or
Rate = rate of disappearance of either reactant.
Rate = - Δ[sucrose] / Δsec (- since want a + rate)
In order to obtain rate, we need a way to measure ΔM of a
reactant or product with respect to time.](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-2-2048.jpg)
![II. Rates
Example: 2 N2O5 -----) 4 NO2 + 1 O2
If we want to equalize the rates then:
Rate = Δ[O2] = 1/4 Δ[NO2] = - 1/2 Δ[N2O5]
Δt Δt Δt
- divide by balancing coefficients when we equalize rates.
Various Rates can be determined: 1) instantaneous rate at a
given time; 2) average rate over a long period of time; or 3) the
initial rate – rate at the beginning of the rxn; ie rate at t=0.0 (this
is used the most).
On next slide the Δ[O2] versus time is plotted for a reaction.
Note: 1) how the rate changes with time & 2) that rate is the tangent
at a given point on the curve.](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-3-2048.jpg)

![III. Rate Law: rate = k x [A]m x [B]n for A + B
Rate Law relates the rate to temperature & concentration.
Rate law is given in terms of REACTANTS only (convention).
k = rate constant & handles the temperature variable.
The exponents are the order & handle the concentration variables.
General form of the rate law for: a A + b B c C + d D
rate = k x [A]m x [B]n
- Order for A is m & order for B is n; Overall order is: m + n
- m & n are determined experimentally.
- k, also determined experimentally & units depend upon overall order.](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-5-2048.jpg)
![III. Rate Law - Rate Constant & Units
Note: Assume time is in seconds (s). Rate = k [A]x
Solve for k & plug in units; k = Rate / [A]x
(this may be useful for one of the online HW problems)
Overall Rxn Order, x Units for k
zero Ms-1
first s-1
second M-1s-1
third M-2s-1](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-6-2048.jpg)
![IV. Order
The concentration variables are handled by the exponents - the order.
The orders are determined experimentally except for one case: An
elementary reaction.
Elementary reactions are one step reactions which are the individual
steps in a mechanism. (For an elementary reaction only: the
balancing coefficients determine the order.) - Important
Example 1 for a multistep reaction: 1 CH2Br2 + 2 KI ---) 1 CH2I2 + 2 KBr
If experimentation found that m & n were both first order; then:
rate = k [CH2Br2 ]1 [KI]1
Example 2 for an elementary reaction: 2 O3 ---) 3 O2 (told it is elementary)
No need for experimentation; order comes from balancing coefficients:
rate = k[O3]2](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-7-2048.jpg)


![IV. Determination of Order by varying M
Example #1: Determine the order for & rate expression for:
2N2O5 ---) 4NO2 + 1O2 rate = k [N2O5]m
Exp #1: Rate = 4.8x10-6 Ms-1 at 1.0x10-2 M N2O5
Exp #2: Rate = 9.6x10-6 Ms-1 at 2.0x10-2 M N2O5
Order: Note that when [N2O5] doubles, the rate doubles. Since
rate α [N2O5]m & rate doubles when [N2O5] doubles, the value of
must be 1; the order is 1.
- rate α [N2O5]m & rate doubles when [N2O5] doubles, then:
go from [1]m = 1 to [2]m = 2 m has to be 1
Rate law: rate = k x [N2O5]1](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-10-2048.jpg)
![IV. Determination of Order by varying M
Summary
EFFECTS of doubling reagent M while keeping others constant:
Rate remains the same 0th order: [M]0
Rate doubles 1st order: [M]1
Rate quadruples 2nd order: [M]2
Rate increases eightfold 3rd order: [M]3](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-11-2048.jpg)
![IV. Determination of Order by varying M
Example #2: 2 NO + Cl2 -----) 2 NOCl - Calculate order of Rxn
Exp Initial [NO] Initial [Cl2] Initial Rate, Ms-1
1 0.0125 0.0255 2.27x10-5
2 0.0125 0.0510 4.57x10-5
3 0.0250 0.0255 9.08x10-5
Rate = k[NO]m[Cl2]n
a) calculate n: From 1 & 2 - double [Cl2] & keep [NO] constant
& rate increases by factor of 2.01; n = 1
b) calculate m: From 1 & 3 - double [NO] & keep [Cl2] constant
& rate increases by factor of 4.00; m = 2
Rate = k[NO]2[Cl2]1
2nd order wrt [NO]; 1st order wrt [Cl2]; 3rd order overall](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-12-2048.jpg)

![IV. Determination of Order by Integrated Rate Expression
Summary on use of logarithms
Log: involves #’s to the base 10; Log 10x = x
Ln: Natural log uses #’s to the base e; Ln ex = x
Ln [A/B] = Ln A - Ln B (or Log)
Ln [A x B] = Ln A + Ln B (or Log)
Ln Ab = b Ln A (or Log)
To obtain either log or ln use the appropriate calculator function.
Log 2.1x10-4 = - 3.68 (note significant figure change - see below)
(-4.0000000…. + 0.32 = -3.68 ; cut off at first doubtful digit)
To remove Ln & Log use the inverse; ex & 10x functions on cal.
Inverse [log 3.00] or 103.00 = 1.0 x 103
Inverse [ln 3.00] or e3.00 = 20.](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-14-2048.jpg)
![IV. Order Integrated Rate Law - First Order Rxns
1) 1st Order Reactions: aA -----) Products If 1st order, then
-Δ[A]/Δt = k[A]1 (rate expression)
- This plot for first order data only gives minimal information
[A]
Time](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-15-2048.jpg)
![IV. Order Integrated Rate Law - First Order Rxns
1) 1st Order Reactions: aA -----) bB -Δ[A]/Δt = k[A]1
- if we integrate from time t to 0, we get the following:
Y = mX + b
ln[A]t = - kt + ln[A]o or ln{[A]t/[A]o} = -kt
where [A]t = M of A at time = t & [A]o = M of A at t = 0
- A plot of ln[A]t versus t gives a straight line (Y = mX +b):
b Slope (m) = - k
Note: Only linear for 1st order
ln[A]t
Time, t](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-16-2048.jpg)
![IV. Order Integrated Rate Law - First Order Rxns
Half-Life (t1/2) of 1st Order Reaction:
t1/2 = time it takes for [A]o to decrease to 1/2 initial M = ½[A]o
ln [A]t /[A]o = -kt ln 1/2[A]o /[A]o = -kt1/2
ln 1/2 = -kt1/2 -0.693 = -kt1/2 t1/2 = 0.693 / k
Note: 1) Time for 1/2 to disappear is independent of [A] for 1st order reaction.
2) This is an easy way to calculate 1st order rate constant, k.
Example: If t1/2 = 189 sec for 1st order decomposition of 1.0
mole of H2O2, then how much H2O2 will be left after 378 sec?
Note: 378/189 = 2 Goes through two half lives
1.0 mol ---) 0.50 mol ---) 0.25 mol](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-17-2048.jpg)
![IV. Order Integrated Rate Law - Second Order Rxns
2) Second Order Reactions:
- Assume that aA -----) Products is 2nd order
Rate = - Δ[A] / Δ t = k [A]2
Integrate rate expression from time t to 0 & get following:
1/[A]t = k t + 1/[A]o So, a plot of 1/[A]t vs t should give a
straight line with slope = k and y intercept = 1/[A]o
t1/2 = 1 Note: Now t1/2 depends on initial M
k x [A]0
Note: can tell if reaction is 2nd order from 1/[A] vs t plot.](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-18-2048.jpg)
![IV. Order Integrated Rate Law - Second Order Rxns Example
Plot of ln[NO2] vs t is not
linear – not 1st order.](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-19-2048.jpg)
![IV. Order Integrated Rate Law - 0th Order
3) 0th Order Reactions Assume A ---) B is 0th order:
Rate = -k[A]0
Rate = -k
- “Integrated” Rate Equation for a 0th order reaction:
[A]t = -k x t + [A]0
- a plot of [A]t versus t will give a straight line
- Again, if you let [A]t = 1/2 [A]o then t = t1/2
- t1/2 = [A]0 / 2k](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-20-2048.jpg)
![IV. Order Integrated Rate Law - Summary
Δ Rate
when
double [M]
None
Double
Quadruple](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-21-2048.jpg)
![IV. Order Integrated Rate Law - Summary
[A]t
Time, t
ln[A]t
Time, t
1/[A]t
Time, t
0th Order n=0
[A]t = - kt + [A]o
1st Order n=1
ln[A]t = - kt + ln[A]o
2th Order n=2
1/[A]t = kt + 1/[A]o
A B Δ[A]/Δt = k[A]n
Note: slope = k in
each case](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-22-2048.jpg)
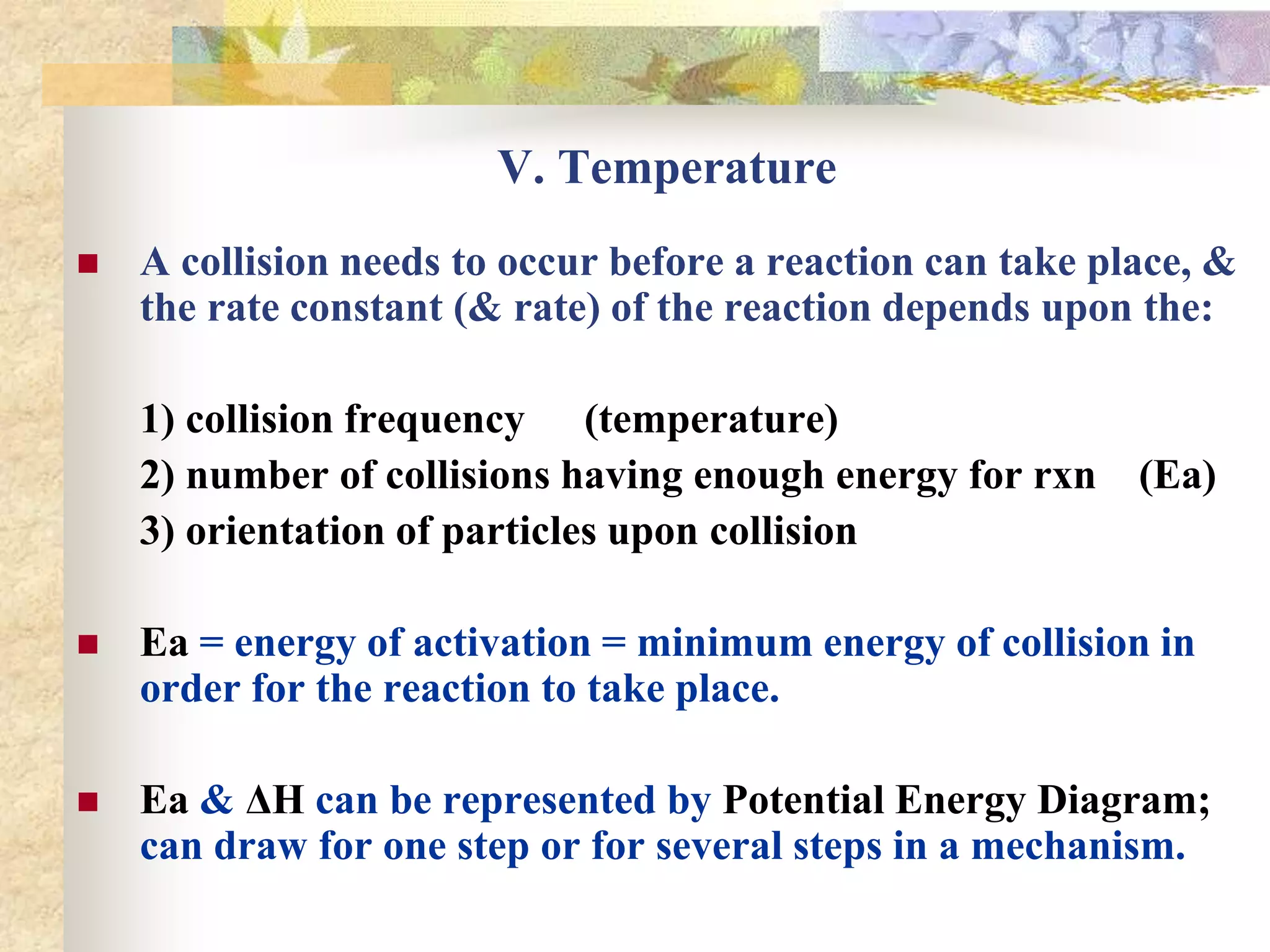


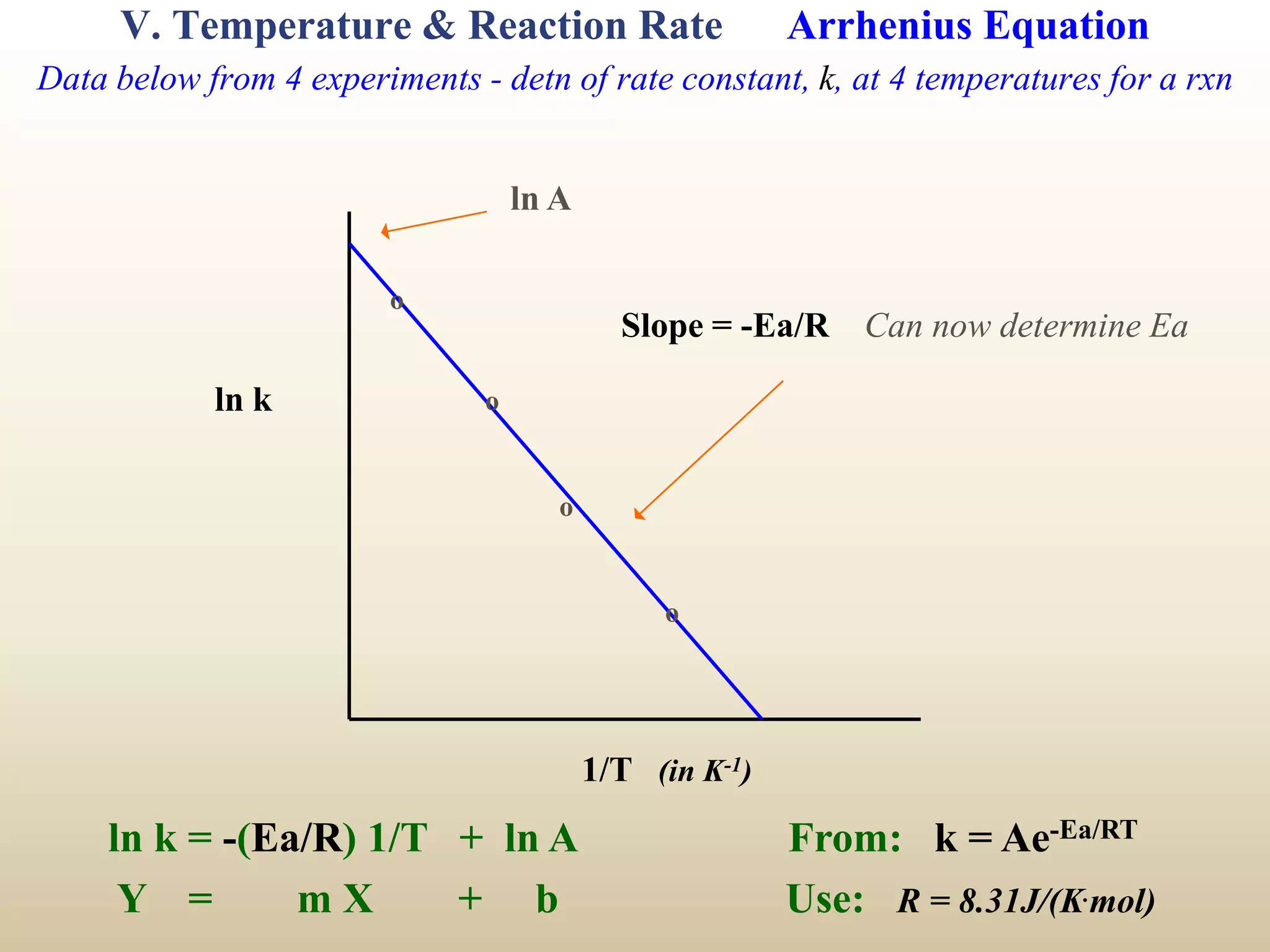
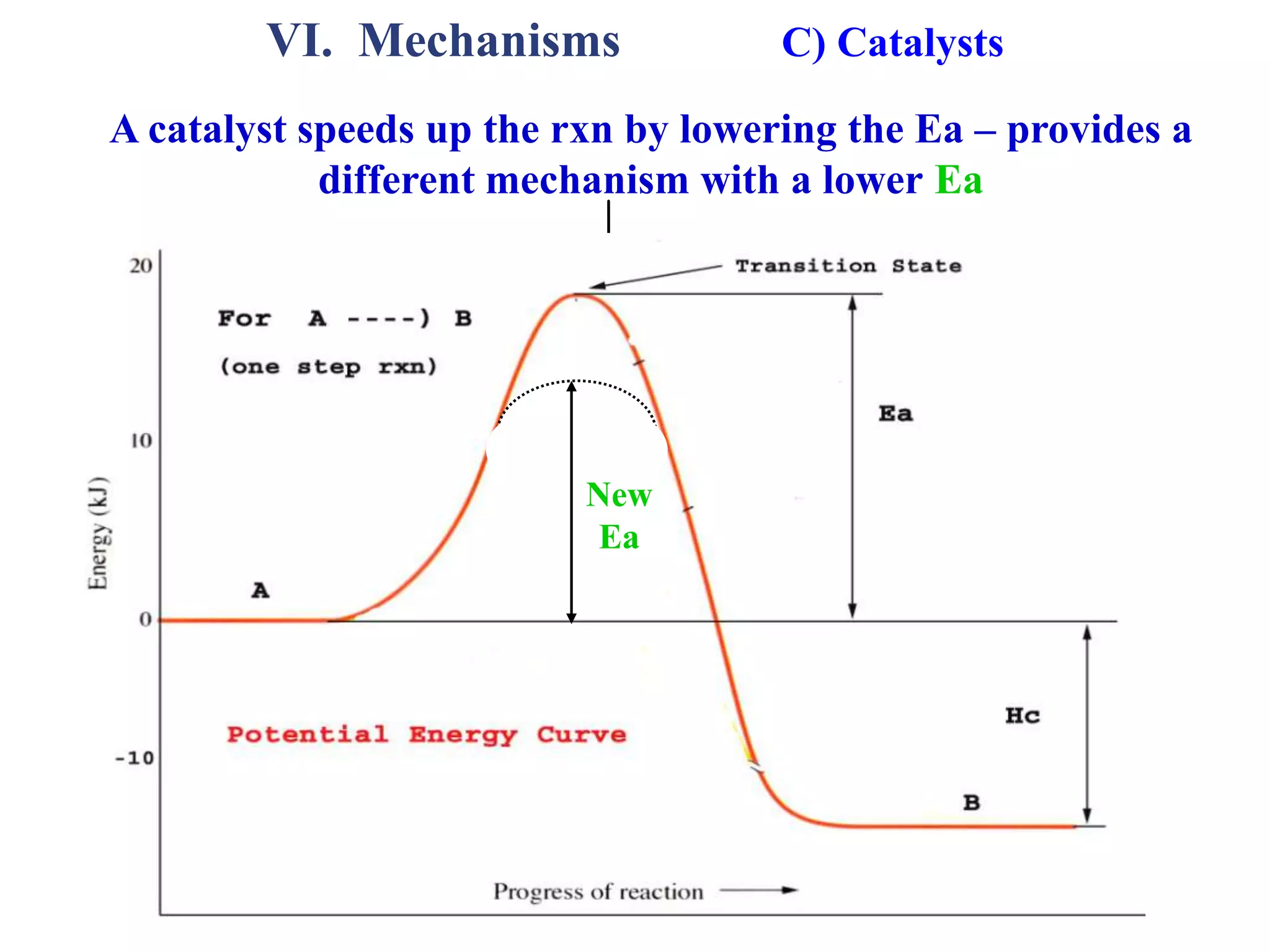
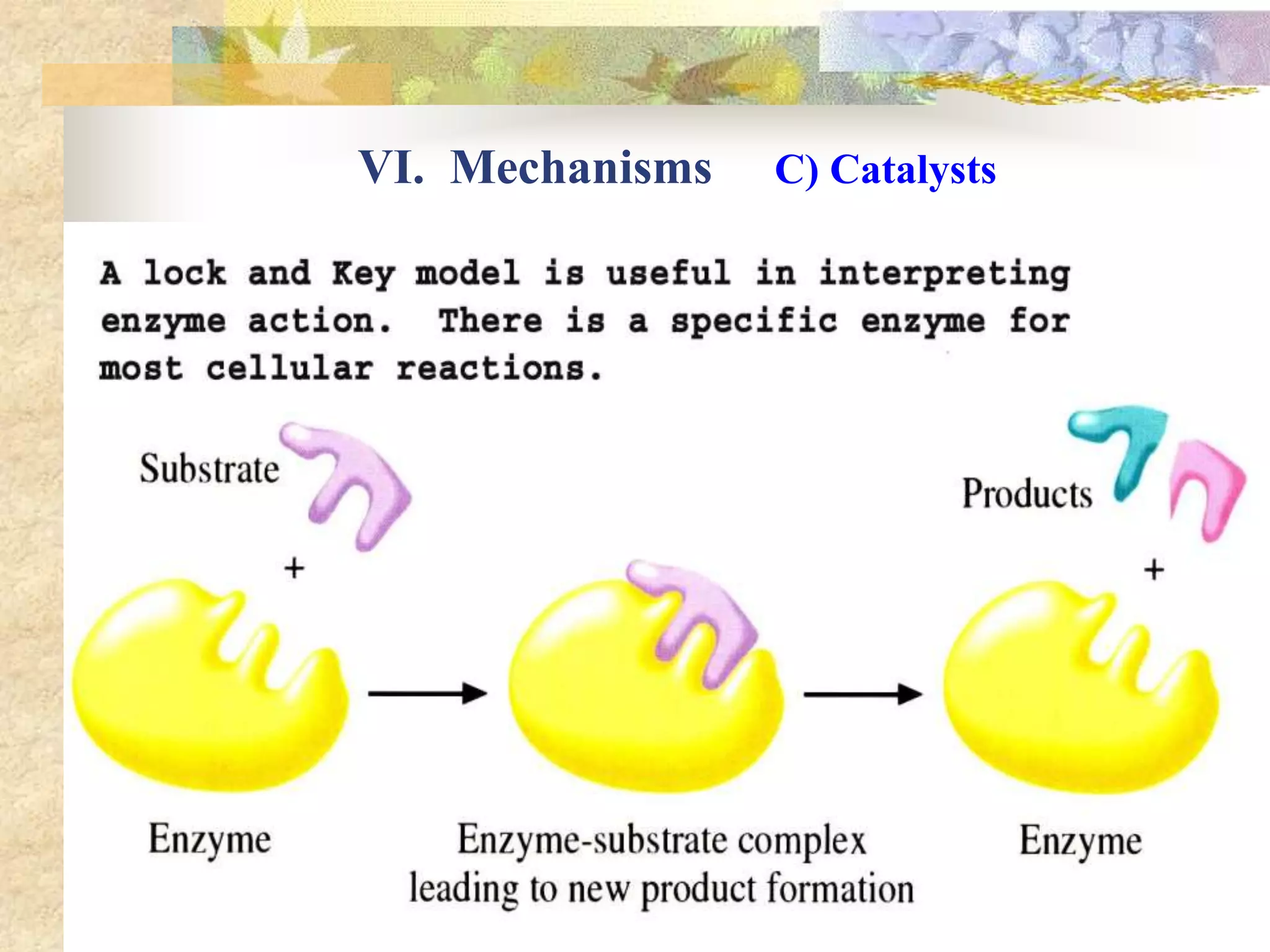
![VI. Mechanisms B) Example 1 - Information
Overall Rxn: O3 + 2NO2 -----) O2 + N2O5
Suggested two-step mechanism (from experimentation):
Step 1) O3 + NO2 -----) NO3 + O2 (slow)
Step 2) NO3 + NO2 -----) N2O5
rate = k [O3]1[NO2]1 - from slow first step
Notes: a) Two elementary reactions (NOTE: balancing
coefficients = orders in an elementary rxn)
b) Steps add up to give overall rxn
c) NO3 is an intermediate (produced & used up)
d) There is no catalyst
e) Slowest step governs the overall rate
mechanism is useful & will give us: a) practical data, b) rate
law, c) theoretical data, d) understanding of reaction](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-28-2048.jpg)
![VI. Mechanisms B) Example 2 - Calculate Rate Expression
Determine a) general rate expression & b) complete rate law from
the following mechanism Note: can directly get the order for an
elementary rxn from the balancing coefficients.
1) 1I2 2Io (fast equilibrium)
2) 2Io + 1H2 2HI (slow)
a) Overall Rxn from addn of steps: 1I2 + 1H2 2HI
General rate law: rate = k[I2]x[H2]y
b) Complete rate expression from mechanism:
From step #2: (rxn rate = slow step rate) rate = k2 [Io]2 x [H2]1
From step #1: Keq = [Io]2/[I2]1 [Io]2 = Keq[I2]1 Substitute into above:
rate = k2 Keq [I2]1 [H2]1
rate = k [I2]1 [H2]1](https://image.slidesharecdn.com/chemicalkinetics-i-140520054239-phpapp01/75/Chemical-kinetics-29-2048.jpg)