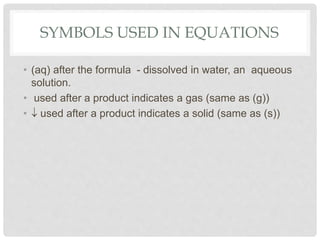
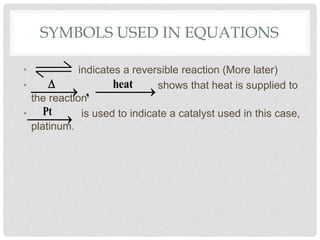
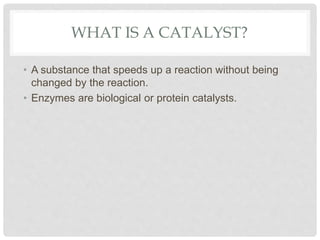
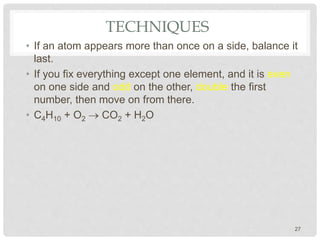
This document discusses balancing chemical equations. It explains that chemical reactions have reactants that turn into products, with the atoms being rearranged but not created or destroyed. Chemical equations use symbols to represent the physical states and show the reactants yielding the products. Balancing chemical equations ensures the same number and type of atoms are on both sides, done by adding coefficients without changing formulas. Examples show balancing equations step-by-step using techniques like balancing the most repeated element last.