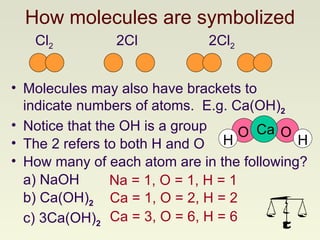
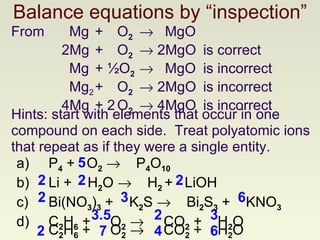
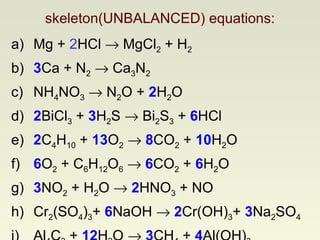
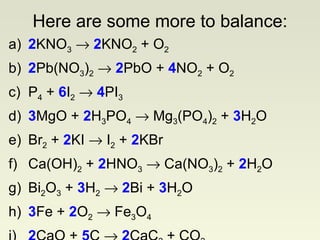
The document discusses techniques for writing and balancing chemical equations, including representing molecules with chemical formulas, determining the number of atoms in different compounds, using conservation of mass to balance equations, and examples of balancing a variety of chemical reactions. It provides guidance on balancing a number of sample equations involving different elements and compounds.