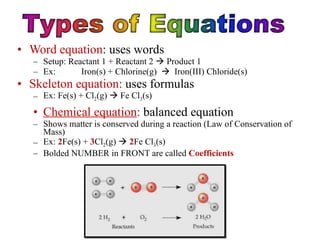
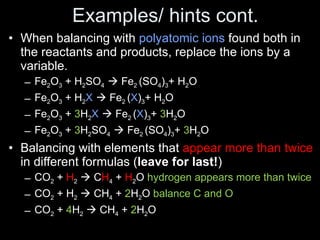
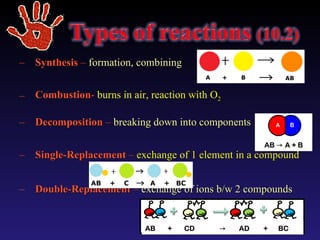
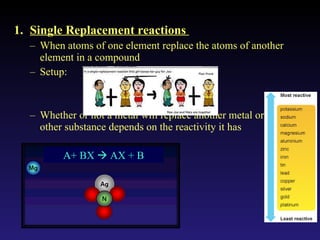
This document discusses chemical reactions. It defines different types of chemical reactions including synthesis, combustion, decomposition, single replacement, and double replacement reactions. It provides examples of each type of reaction and gives steps for writing and balancing chemical equations. Key points covered include identifying evidence of a chemical reaction, writing word and skeleton equations, and using coefficients to balance equations so matter is conserved.